Cystathionine beta-synthase mutants exhibit changes in protein unfolding: conformational analysis of misfolded variants in crude cell extracts
- PMID: 22069143
- PMCID: PMC3319881
- DOI: 10.1007/s10545-011-9407-4
Cystathionine beta-synthase mutants exhibit changes in protein unfolding: conformational analysis of misfolded variants in crude cell extracts
Abstract
Protein misfolding has been proposed to be a common pathogenic mechanism in many inborn errors of metabolism including cystathionine β-synthase (CBS) deficiency. In this work, we describe the structural properties of nine CBS mutants that represent a common molecular pathology in the CBS gene. Using thermolysin in two proteolytic techniques, we examined conformation of these mutants directly in crude cell extracts after expression in E. coli. Proteolysis with thermolysin under native conditions appeared to be a useful technique even for very unstable mutant proteins, whereas pulse proteolysis in a urea gradient had limited values for the study of the majority of CBS mutants due to their instability. Mutants in the active core had either slightly increased unfolding (p.A114V, p.E302K and p.G307S) or extensive unfolding with decreased stability (p.H65R, p.T191M, p.I278T and p.R369C). The extent of the unfolding inversely correlated with the previously determined degree of tetrameric assembly and with the catalytic activity. In contrast, mutants bearing aminoacid substitutions in the C-terminal regulatory domain (p.R439Q and p.D444N) had increased global stability with decreased flexibility. This study shows that proteolytic techniques can reveal conformational abnormalities even for CBS mutants that have activity and/or a degree of assembly similar to the wild-type enzyme. We present here a methodological strategy that may be used in cell lysates to evaluate properties of proteins that tend to misfold and aggregate and that may be important for conformational studies of disease-causing mutations in the field of inborn errors of metabolism.
Figures

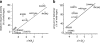
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

