Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes
- PMID: 22069312
- PMCID: PMC3281696
- DOI: 10.1074/jbc.M111.277178
Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes
Abstract
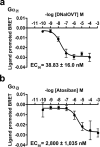
We used a bioluminescence resonance energy transfer biosensor to screen for functional selective ligands of the human oxytocin (OT) receptor. We demonstrated that OT promoted the direct engagement and activation of G(q) and all the G(i/o) subtypes at the OT receptor. Other peptidic analogues, chosen because of specific substitutions in key OT structural/functional residues, all showed biased activation of G protein subtypes. No ligand, except OT, activated G(oA) or G(oB), and, with only one exception, all of the peptides that activated G(q) also activated G(i2) and G(i3) but not G(i1), G(oA), or G(oB), indicating a strong bias toward these subunits. Two peptides (DNalOVT and atosiban) activated only G(i1) or G(i3), failed to recruit β-arrestins, and did not induce receptor internalization, providing the first clear examples of ligands differentiating individual G(i/o) family members. Both analogs inhibited cell proliferation, showing that a single G(i) subtype-mediated pathway is sufficient to prompt this physiological response. These analogs represent unique tools for examining the contribution of G(i/o) members in complex biological responses and open the way to the development of drugs with peculiar selectivity profiles. This is of particular relevance because OT has been shown to improve symptoms in neurodevelopmental and psychiatric disorders characterized by abnormal social behaviors, such as autism. Functional selective ligands, activating a specific G protein signaling pathway, may possess a higher efficacy and specificity on OT-based therapeutics.
Figures


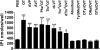


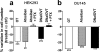
References
-
- Urban J. D., Clarke W. P., von Zastrow M., Nichols D. E., Kobilka B., Weinstein H., Javitch J. A., Roth B. L., Christopoulos A., Sexton P. M., Miller K. J., Spedding M., Mailman R. B. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13 - PubMed
-
- Zhou X. B., Lutz S., Steffens F., Korth M., Wieland T. (2007) Oxytocin receptors differentially signal via Gq and Gi proteins in pregnant and nonpregnant rat uterine myocytes: implications for myometrial contractility. Mol. Endocrinol. 21, 740–752 - PubMed
-
- Strakova Z., Soloff M. S. (1997) Coupling of oxytocin receptor to G proteins in rat myometrium during labor: Gi receptor interaction. Am. J. Physiol. 272, E870–E876 - PubMed
-
- Gravati M., Busnelli M., Bulgheroni E., Reversi A., Spaiardi P., Parenti M., Toselli M., Chini B. (2010) Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. J. Neurochem. 114, 1424–1435 - PubMed
-
- Rimoldi V., Reversi A., Taverna E., Rosa P., Francolini M., Cassoni P., Parenti M., Chini B. (2003) Oxytocin receptor elicits different EGFR/MAPK activation patterns depending on its localization in caveolin-1 enriched domains. Oncogene 22, 6054–6060 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

