Pseudomonas aeruginosa elastase provides an escape from phagocytosis by degrading the pulmonary surfactant protein-A
- PMID: 22069491
- PMCID: PMC3206073
- DOI: 10.1371/journal.pone.0027091
Pseudomonas aeruginosa elastase provides an escape from phagocytosis by degrading the pulmonary surfactant protein-A
Abstract
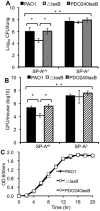
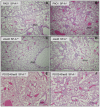
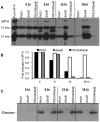
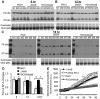
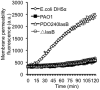
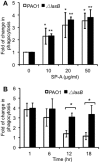
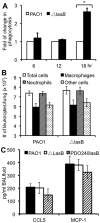
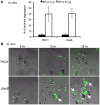
Pseudomonas aeruginosa is an opportunistic pathogen that causes both acute pneumonitis in immunocompromised patients and chronic lung infections in individuals with cystic fibrosis and other bronchiectasis. Over 75% of clinical isolates of P. aeruginosa secrete elastase B (LasB), an elastolytic metalloproteinase that is encoded by the lasB gene. Previously, in vitro studies have demonstrated that LasB degrades a number of components in both the innate and adaptive immune systems. These include surfactant proteins, antibacterial peptides, cytokines, chemokines and immunoglobulins. However, the contribution of LasB to lung infection by P. aeruginosa and to inactivation of pulmonary innate immunity in vivo needs more clarification. In this study, we examined the mechanisms underlying enhanced clearance of the ΔlasB mutant in mouse lungs. The ΔlasB mutant was attenuated in virulence when compared to the wild-type strain PAO1 during lung infection in SP-A+/+ mice. However, the ΔlasB mutant was as virulent as PAO1 in the lungs of SP-A⁻/⁻ mice. Detailed analysis showed that the ΔlasB mutant was more susceptible to SP-A-mediated opsonization but not membrane permeabilization. In vitro and in vivo phagocytosis experiments revealed that SP-A augmented the phagocytosis of ΔlasB mutant bacteria more efficiently than the isogenic wild-type PAO1. The ΔlasB mutant was found to have a severely reduced ability to degrade SP-A, consequently making it unable to evade opsonization by the collectin during phagocytosis. These results suggest that P. aeruginosa LasB protects against SP-A-mediated opsonization by degrading the collectin.
Conflict of interest statement
Figures


References
-
- Clements JA. Surface tension of lung extracts. Proc Soc Exp Biol Med. 1957;95:170–172. - PubMed
-
- Crouch E, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. - PubMed
-
- Hawgood S, Shiffer K. Structures and properties of the surfactant-associated proteins. Annu Rev Physiol. 1991;53:375–394. - PubMed
-
- Holmskov U, Thiel S, Jensenius JC. Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. - PubMed
-
- Pattle RE. Properties, function and origin of the lining layer. Nature. 1955;175:1125–1126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

