Cell-associated flagella enhance the protection conferred by mucosally-administered attenuated Salmonella Paratyphi A vaccines
- PMID: 22069504
- PMCID: PMC3206010
- DOI: 10.1371/journal.pntd.0001373
Cell-associated flagella enhance the protection conferred by mucosally-administered attenuated Salmonella Paratyphi A vaccines
Expression of concern in
-
Expression of Concern: Cell-Associated Flagella Enhance the Protection Conferred by Mucosally-Administered Attenuated Salmonella Paratyphi A Vaccines.PLoS Negl Trop Dis. 2024 May 6;18(5):e0012160. doi: 10.1371/journal.pntd.0012160. eCollection 2024 May. PLoS Negl Trop Dis. 2024. PMID: 38709719 Free PMC article. No abstract available.
Abstract
Background: Antibiotic-resistant Salmonella enterica serovar Paratyphi A, the agent of paratyphoid A fever, poses an emerging public health dilemma in endemic areas of Asia and among travelers, as there is no licensed vaccine. Integral to our efforts to develop a S. Paratyphi A vaccine, we addressed the role of flagella as a potential protective antigen by comparing cell-associated flagella with exported flagellin subunits expressed by attenuated strains.
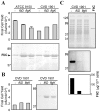
Methodology: S. Paratyphi A strain ATCC 9150 was first deleted for the chromosomal guaBA locus, creating CVD 1901. Further chromosomal deletions in fliD (CVD 1901D) or flgK (CVD 1901K) were then engineered, resulting in the export of unpolymerized FliC, without impairing its overall expression. The virulence of the resulting isogenic strains was examined using a novel mouse LD(50) model to accommodate the human-host restricted S. Paratyphi A. The immunogenicity of the attenuated strains was then tested using a mouse intranasal model, followed by intraperitoneal challenge with wildtype ATCC 9150.
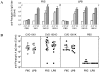
Results: Mucosal (intranasal) immunization of mice with strain CVD 1901 expressing cell-associated flagella conferred superior protection (vaccine efficacy [VE], 90%) against a lethal intraperitoneal challenge, compared with the flagellin monomer-exporting mutants CVD 1901K (30% VE) or CVD 1901D (47% VE). The superior protection induced by CVD 1901 with its cell-attached flagella was associated with an increased IgG2a:IgG1 ratio of FliC-specific antibodies with enhanced opsonophagocytic capacity.
Conclusions: Our results clearly suggest that enhanced anti-FliC antibody-mediated clearance of S. Paratyphi A by phagocytic cells, induced by vaccines expressing cell-associated rather than exported FliC, might be contributing to the vaccine-induced protection from S. Paratyphi A challenge in vivo. We speculate that an excess of IgG1 anti-FliC antibodies induced by the exported FliC may compete with the IgG2a subtype and block binding to specific phagocyte Fc receptors that are critical for clearing an S. Paratyphi A infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Vollaard AM, Ali S, Widjaja S, Asten HA, Visser LG, et al. Identification of typhoid fever and paratyphoid fever cases at presentation in outpatient clinics in Jakarta, Indonesia. Trans R Soc Trop Med Hyg. 2005;99:440–450. - PubMed
-
- Karkey A, Aryjal A, Basnyat B, Baker S. Kathmandu, Nepal: still an enteric fever capital of the world. J Infect Dev Ctries. 2008;2:461–465. - PubMed
-
- Gupta SK, Medalla F, Omondi MW, Whichard JM, Fields PI, et al. Laboratory-based surveillance of paratyphoid fever in the United States: travel and antimicrobial resistance. Clin Infect Dis. 2008;46:1656–1663. - PubMed
-
- Levine MM, Taylor DN, Ferreccio C. Typhoid vaccines come of age. Pediatr Infect Dis J. 1989;8:374–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

