Spectrin-adducin membrane skeleton: A missing link between epithelial junctions and the actin cytoskeletion?
- PMID: 22069512
- PMCID: PMC3210521
- DOI: 10.4161/bioa.1.4.17642
Spectrin-adducin membrane skeleton: A missing link between epithelial junctions and the actin cytoskeletion?
Abstract
Adherens junctions (AJs) and tight junctions (TJs) represent key adhesive structures that regulate the apico-basal polarity and barrier properties of epithelial layers. AJs and TJs readily undergo disassembly and reassembly during normal tissue remodeling and disruption of epithelial barriers in diseases. Such junctional plasticity depends on the orchestrated dynamics of the plasma membrane with its underlying F-actin cytoskeleton, however the interplay between these cellular structures remains poorly understood. Recent studies highlighted the spectrin-adducin-based membrane skeleton as an emerging regulator of AJ and TJ integrity and remodeling. Here we discuss new evidences implicating adducin, spectrin and other membrane skeleton proteins in stabilization of epithelial junctions and regulation of junctional dynamics. Based on the known ability of the membrane skeleton to link cortical actin filaments to the plasma membrane, we hypothesize that the spectrin-adducin network serves as a critical signal and force transducer from the actomyosin cytoskeleton to junctions during remodeling of AJs and TJs.
Figures
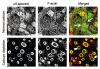
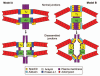
Similar articles
-
Adducins regulate remodeling of apical junctions in human epithelial cells.Mol Biol Cell. 2010 Oct 15;21(20):3506-17. doi: 10.1091/mbc.E10-03-0259. Epub 2010 Sep 1. Mol Biol Cell. 2010. PMID: 20810786 Free PMC article.
-
Epithelial-specific isoforms of protein 4.1R promote adherens junction assembly in maturing epithelia.J Biol Chem. 2020 Jan 3;295(1):191-211. doi: 10.1074/jbc.RA119.009650. Epub 2019 Nov 27. J Biol Chem. 2020. PMID: 31776189 Free PMC article.
-
Actin-interacting protein 1 controls assembly and permeability of intestinal epithelial apical junctions.Am J Physiol Gastrointest Liver Physiol. 2015 May 1;308(9):G745-56. doi: 10.1152/ajpgi.00446.2014. Epub 2015 Mar 19. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25792565 Free PMC article.
-
Unique and redundant functions of cytoplasmic actins and nonmuscle myosin II isoforms at epithelial junctions.Ann N Y Acad Sci. 2022 Sep;1515(1):61-74. doi: 10.1111/nyas.14808. Epub 2022 Jun 7. Ann N Y Acad Sci. 2022. PMID: 35673768 Free PMC article. Review.
-
Actin motors that drive formation and disassembly of epithelial apical junctions.Front Biosci. 2008 May 1;13:6662-81. doi: 10.2741/3180. Front Biosci. 2008. PMID: 18508686 Review.
Cited by
-
Rho GTPases and actomyosin: Partners in regulating epithelial cell-cell junction structure and function.Exp Cell Res. 2017 Sep 1;358(1):20-30. doi: 10.1016/j.yexcr.2017.03.053. Epub 2017 Mar 29. Exp Cell Res. 2017. PMID: 28363828 Free PMC article. Review.
-
Build them up and break them down: Tight junctions of cell lines expressing typical hepatocyte polarity with a varied repertoire of claudins.Tissue Barriers. 2013 Oct 1;1(4):e25210. doi: 10.4161/tisb.25210. Epub 2013 Jun 4. Tissue Barriers. 2013. PMID: 24665408 Free PMC article.
-
BioArchitecture: the organization and regulation of biological space.Bioarchitecture. 2012 Nov-Dec;2(6):200-3. doi: 10.4161/bioa.22726. Bioarchitecture. 2012. PMID: 23267413 Free PMC article. Review.
-
Regulation of Platelet Derived Growth Factor Signaling by Leukocyte Common Antigen-related (LAR) Protein Tyrosine Phosphatase: A Quantitative Phosphoproteomics Study.Mol Cell Proteomics. 2016 Jun;15(6):1823-36. doi: 10.1074/mcp.M115.053652. Epub 2016 Apr 13. Mol Cell Proteomics. 2016. PMID: 27074791 Free PMC article.
-
F-actin binding protein, anillin, regulates integrity of intercellular junctions in human epithelial cells.Cell Mol Life Sci. 2015 Aug;72(16):3185-3200. doi: 10.1007/s00018-015-1890-6. Epub 2015 Mar 27. Cell Mol Life Sci. 2015. PMID: 25809162 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
