The hepatitis C virus glycan shield and evasion of the humoral immune response
- PMID: 22069522
- PMCID: PMC3205388
- DOI: 10.3390/v3101909
The hepatitis C virus glycan shield and evasion of the humoral immune response
Abstract
Despite the induction of effective immune responses, 80% of hepatitis C virus (HCV)-infected individuals progress from acute to chronic hepatitis. In contrast to the cellular immune response, the role of the humoral immune response in HCV clearance is still subject to debate. Indeed, HCV escapes neutralizing antibodies in chronically infected patients and reinfection has been described in human and chimpanzee. Studies of antibody-mediated HCV neutralization have long been hampered by the lack of cell-culture-derived virus and the absence of a small animal model. However, the development of surrogate models and recent progress in HCV propagation in vitro now enable robust neutralization assays to be performed. These advances are beginning to shed some light on the mechanisms of HCV neutralization. This review summarizes the current state of knowledge of the viral targets of anti-HCV-neutralizing antibodies and the mechanisms that enable HCV to evade the humoral immune response. The recent description of the HCV glycan shield that reduces the immunogenicity of envelope proteins and masks conserved neutralizing epitopes at their surface constitutes the major focus of this review.
Keywords: N-glycosylation; hepatitis C virus; neutralizing antibodies; viral escape.
Figures
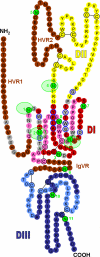
References
-
- Lemon SM, Walker C, Alter MJ, Yi M. Hepatitis c virus. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 1253–1304.
-
- André P, Perlemuter G, Budkowska A, Brechot C, Lotteau V. Hepatitis c virus particles and lipoprotein metabolism. Semin Liver Dis. 2005;25:93–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

