Mechanisms of Cisplatin nephrotoxicity
- PMID: 22069563
- PMCID: PMC3153174
- DOI: 10.3390/toxins2112490
Mechanisms of Cisplatin nephrotoxicity
Abstract
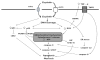
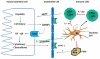
Cisplatin is a widely used and highly effective cancer chemotherapeutic agent. One of the limiting side effects of cisplatin use is nephrotoxicity. Research over the past 10 years has uncovered many of the cellular mechanisms which underlie cisplatin-induced renal cell death. It has also become apparent that inflammation provoked by injury to renal epithelial cells serves to amplify kidney injury and dysfunction in vivo. This review summarizes recent advances in our understanding of cisplatin nephrotoxicity and discusses how these advances might lead to more effective prevention.
Keywords: apoptosis; cisplatin; cytokines; dendritic cells; inflammation; kidney injury; nephrotoxicity; toll-like receptors.
Figures
References
-
- Hartmann J.T., Fels L.M., Knop S., Stolt H., Kanz L., Bokemeyer C. A randomized trial comparing the nephrotoxicity of cisplatin/ifosfamide-based combination chemotherapy with or without amifostine in patients with solid tumors. Invest. New Drugs. 2000;18:281–289. - PubMed
-
- Hartmann J.T., Lipp H.-P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003;4:889–901. - PubMed
-
- Sastry J., Kellie S.J. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr. Hematol. Oncol. 2005;22:441–445. - PubMed
-
- Arany I., Safirstein R.L. Cisplatin nephrotoxicity. Semin. Nephrol. 2003;23:460–464. - PubMed
-
- Boulikas T. Poly(ADP-ribose) synthesis in blocked and damaged cells and its relation to carcinogens. Anticancer Res. 1992;12:885–898. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

