Toxin-based therapeutic approaches
- PMID: 22069564
- PMCID: PMC3153180
- DOI: 10.3390/toxins2112519
Toxin-based therapeutic approaches
Abstract
Protein toxins confer a defense against predation/grazing or a superior pathogenic competence upon the producing organism. Such toxins have been perfected through evolution in poisonous animals/plants and pathogenic bacteria. Over the past five decades, a lot of effort has been invested in studying their mechanism of action, the way they contribute to pathogenicity and in the development of antidotes that neutralize their action. In parallel, many research groups turned to explore the pharmaceutical potential of such toxins when they are used to efficiently impair essential cellular processes and/or damage the integrity of their target cells. The following review summarizes major advances in the field of toxin based therapeutics and offers a comprehensive description of the mode of action of each applied toxin.
Keywords: anthrax; diphtheria toxin; immunotoxins; pseudomonas exotoxin A; ricin; suicide gene; targeting; toxins.
Figures
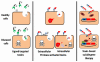



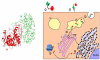

References
-
- Kostrzewa R., Segura-Aguilar J. Botulinum neurotoxin: Evolution from poison, to research tool—onto medicinal therapeutic and future pharmaceutical panacea. Neurotox. Res. 2007;12:275–290. - PubMed
-
- Mahajan S.T., Brubaker L. Botulinum toxin: From life-threatening disease to novel medical therapy. Am. J. Obstet. Gynecol. 2007;196:7–15. - PubMed
-
- Erbguth F.J. From poison to remedy: The chequered history of botulinum toxin. J. Neur. Transm. 2008;115:559–565. - PubMed
-
- Truong D.D., Stenner A., Reichel G. Current Clinical Applications of Botulinum Toxin. Curr. Pharm. Design. 2009;15:3671–3680. - PubMed
-
- Winau F., Westphal O., Winau R. Paul Ehrlich—In search of the magic bullet. Microb. Infect. 2004;6:786–789. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

