Proteases as insecticidal agents
- PMID: 22069618
- PMCID: PMC3153225
- DOI: 10.3390/toxins2050935
Proteases as insecticidal agents
Abstract
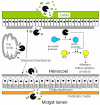
Proteases from a variety of sources (viruses, bacteria, fungi, plants, and insects) have toxicity towards insects. Some of these insecticidal proteases evolved as venom components, herbivore resistance factors, or microbial pathogenicity factors, while other proteases play roles in insect development or digestion, but exert an insecticidal effect when over-expressed from genetically engineered plants or microbial pathogens. Many of these proteases are cysteine proteases, although insect-toxic metalloproteases and serine proteases have also been examined. The sites of protease toxic activity range from the insect midgut to the hemocoel (body cavity) to the cuticle. This review discusses these insecticidal proteases along with their evaluation and use as potential pesticides.
Keywords: basement membrane; cuticle; insecticides; microbial defense; peritrophic matrix; plant defense.
Figures

References
-
- Devine G.J., Furlong M.J. Insecticide use: Contexts and ecological consequences. Agric. Human Values. 2007;24:281–306. doi: 10.1007/s10460-007-9067-z. - DOI
-
- Brookes G., Barfoot P. Global impact of biotech crops: Socio-economic and environmental effects, 1996-2006. AgBioForum. 2008;11:21–38.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

