Toxin-specific antibodies for the treatment of Clostridium difficile: current status and future perspectives
- PMID: 22069622
- PMCID: PMC3153223
- DOI: 10.3390/toxins2050998
Toxin-specific antibodies for the treatment of Clostridium difficile: current status and future perspectives
Abstract
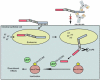
Therapeutic agents targeting bacterial virulence factors are gaining interest as non-antibiotic alternatives for the treatment of infectious diseases. Clostridium difficile is a Gram-positive pathogen that produces two primary virulence factors, enterotoxins A and B (TcdA and TcdB), which are responsible for Clostridium difficile-associated disease (CDAD) and are targets for CDAD therapy. Antibodies specific for TcdA and TcdB have been shown to effectively treat CDAD and prevent disease relapse in animal models and in humans. This review summarizes the various toxin-specific antibody formats and strategies under development, and discusses future directions for CDAD immunotherapy, including the use of engineered antibody fragments with robust biophysical properties for systemic and oral delivery.
Keywords: Clostridium difficile; Clostridium difficile-associated disease; antibody; neutralization; single-domain antibody; therapy; toxin.
Figures

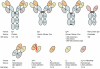
Similar articles
-
Antibody against TcdB, but not TcdA, prevents development of gastrointestinal and systemic Clostridium difficile disease.J Infect Dis. 2013 Jan 15;207(2):323-30. doi: 10.1093/infdis/jis669. Epub 2012 Nov 2. J Infect Dis. 2013. PMID: 23125448 Free PMC article.
-
Neutralization of Clostridium difficile toxin A with single-domain antibodies targeting the cell receptor binding domain.J Biol Chem. 2011 Mar 18;286(11):8961-76. doi: 10.1074/jbc.M110.198754. Epub 2011 Jan 7. J Biol Chem. 2011. PMID: 21216961 Free PMC article.
-
Defining the Roles of TcdA and TcdB in Localized Gastrointestinal Disease, Systemic Organ Damage, and the Host Response during Clostridium difficile Infections.mBio. 2015 Jun 2;6(3):e00551. doi: 10.1128/mBio.00551-15. mBio. 2015. PMID: 26037121 Free PMC article.
-
Against Clostridioides difficile Infection: An Update on Vaccine Development.Toxins (Basel). 2025 May 1;17(5):222. doi: 10.3390/toxins17050222. Toxins (Basel). 2025. PMID: 40423305 Free PMC article. Review.
-
Hyperimmune bovine colostrum for treatment of GI infections: a review and update on Clostridium difficile.Hum Vaccin Immunother. 2013 Jul;9(7):1565-8. doi: 10.4161/hv.24078. Epub 2013 Feb 22. Hum Vaccin Immunother. 2013. PMID: 23435084 Review.
Cited by
-
The Murine Neonatal Fc Receptor Is Required for Transport of Immunization-Induced C. difficile-Specific IgG to the Gut and Protection against Disease but Does Not Affect Disease Susceptibility.Infect Immun. 2021 Sep 16;89(10):e0027421. doi: 10.1128/IAI.00274-21. Epub 2021 Jun 7. Infect Immun. 2021. PMID: 34097471 Free PMC article.
-
Structural basis for antibody recognition in the receptor-binding domains of toxins A and B from Clostridium difficile.J Biol Chem. 2014 Jan 24;289(4):2331-43. doi: 10.1074/jbc.M113.505917. Epub 2013 Dec 5. J Biol Chem. 2014. PMID: 24311789 Free PMC article.
-
Using phenotype microarrays to determine culture conditions that induce or repress toxin production by Clostridium difficile and other microorganisms.PLoS One. 2013;8(2):e56545. doi: 10.1371/journal.pone.0056545. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437164 Free PMC article.
-
Clostridium difficile infection: molecular pathogenesis and novel therapeutics.Expert Rev Anti Infect Ther. 2014 Jan;12(1):131-50. doi: 10.1586/14787210.2014.866515. Expert Rev Anti Infect Ther. 2014. PMID: 24410618 Free PMC article. Review.
-
New Insights into Clostridium difficile (CD) Infection in Latin America: Novel Description of Toxigenic Profiles of Diarrhea-Associated to CD in Bogotá, Colombia.Front Microbiol. 2018 Jan 30;9:74. doi: 10.3389/fmicb.2018.00074. eCollection 2018. Front Microbiol. 2018. PMID: 29441053 Free PMC article.
References
-
- Rupnik M., Wilcox M.H., Gerding D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009;7:526–536. - PubMed
-
- O’Connor J.R., Johnson S., Gerding D.N. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913–1924. - PubMed
-
- McDonald L.C., Killgore G.E., Thompson A., Owens R.C. Jr., Kazakova S.V., Sambol S.P., Johnson S., Gerding D.N. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 2005;353:2433–2441. - PubMed
-
- Warny M., Pepin J., Fang A., Killgore G., Thompson A., Brazier J., Frost E., McDonald L.C. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

