Exfoliative toxins of Staphylococcus aureus
- PMID: 22069631
- PMCID: PMC3153237
- DOI: 10.3390/toxins2051148
Exfoliative toxins of Staphylococcus aureus
Abstract
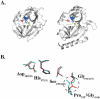
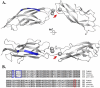
Staphylococcus aureus is an important pathogen of humans and livestock. It causes a diverse array of diseases, ranging from relatively harmless localized skin infections to life-threatening systemic conditions. Among multiple virulence factors, staphylococci secrete several exotoxins directly associated with particular disease symptoms. These include toxic shock syndrome toxin 1 (TSST-1), enterotoxins, and exfoliative toxins (ETs). The latter are particularly interesting as the sole agents responsible for staphylococcal scalded skin syndrome (SSSS), a disease predominantly affecting infants and characterized by the loss of superficial skin layers, dehydration, and secondary infections. The molecular basis of the clinical symptoms of SSSS is well understood. ETs are serine proteases with high substrate specificity, which selectively recognize and hydrolyze desmosomal proteins in the skin. The fascinating road leading to the discovery of ETs as the agents responsible for SSSS and the characterization of the molecular mechanism of their action, including recent advances in the field, are reviewed in this article.
Keywords: Staphylococcus aureus; bullous impetigo; epidermolytic toxin; exfoliative toxin; staphylococcal scalded skin syndrome.
Figures

References
-
- Proft T. In Microbial Toxins: Current Research and Future Trends. Caister Academic Press; Norfolk, UK: 2009. pp. 147–166.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

