Staphylococcal superantigen (TSST-1) mutant analysis reveals that t cell activation is required for biological effects in the rabbit including the cytokine storm
- PMID: 22069685
- PMCID: PMC3153295
- DOI: 10.3390/toxins2092272
Staphylococcal superantigen (TSST-1) mutant analysis reveals that t cell activation is required for biological effects in the rabbit including the cytokine storm
Abstract
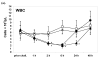
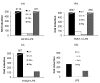
Staphylococcal superantigens (sAgs), such as toxic shock syndrome toxin 1 (TSST-1), induce massive cytokine production, which may result in toxic shock syndrome (TSS) and sepsis. Recently, we reported that in vitro studies in human peripheral blood mononuclear cells (PBMC) do not reflect the immunological situation of the host, because after exposure to superantigens (sAgs) in vivo, mononuclear cells (MNC) leave the circulation and migrate to organs, e.g., the spleen, liver and lung. Our experimental model of choice is the rabbit because it is comparable to humans in its sensitivity to sAg. T cell activation has been assessed by lymphocyte proliferation and IL-2 gene expression after in vivo challenge with TSST-1 and the mutant antigens; expression of the genes of proinflammatory cytokines were taken as indicators for the inflammatory reaction after the combined treatment with TSST-1 and LPS. The question as to whether the biological activities of TSST-1, e.g., lymphocyte extravasation, toxicity and increased sensitivity to LPS, are mediated by T cell activation or activation by MHC II-only, are unresolved and results are contradictory. We have addressed this question by studying these reactions in vivo, with two TSST-1 mutants: one mutated at the MHC binding site (G31R) with reduced MHC binding with residual activity still present, and the other at the T cell binding site (H135A) with no residual function detectable. Here, we report that the mutant G31R induced all the biological effects of the wild type sAg, while the mutant with non-functional TCR binding did not retain any of the toxic effects, proving the pivotal role of T cells in this system.
Keywords: T cell involvement; endotoxin; inflammation; sepsis; superantigens.
Figures








References
-
- Faulkner L., Cooper A., Fantino C., Altman D.M., Sriskandan S. The mechanism of superantigen-mediated toxic shock: not a simple Th1 cytokine storm. J. Immunol. 2005;175:6870–6877. - PubMed
-
- Sriskandan S., Faulkner L., Hopkins P. Streptococcus pyogenes: Insight into the function of the streptococcal superantigens. Int. J. Biochem. Cell Biol. 2007;39:12–19. - PubMed
-
- Sriskandan S., Unnikrishnan M., Krausz T., Dewchand H., van Norden S., Cohen J., Altmann D.M. Enhanced susceptibility to superantigen-associated streptococcal sepsis in human leukocyte antigen-DQ transgenic mice. J. Infect. Dis. 2001;184:166–173. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

