The biology of the cytolethal distending toxins
- PMID: 22069704
- PMCID: PMC3202825
- DOI: 10.3390/toxins3030172
The biology of the cytolethal distending toxins
Abstract
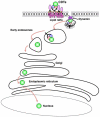
The cytolethal distending toxins (CDTs), produced by a variety of Gram-negative pathogenic bacteria, are the first bacterial genotoxins described, since they cause DNA damage in the target cells. CDT is an A-B(2) toxin, where the CdtA and CdtC subunits are required to mediate the binding on the surface of the target cells, allowing internalization of the active CdtB subunit, which is functionally homologous to the mammalian deoxyribonuclease I. The nature of the surface receptor is still poorly characterized, however binding of CDT requires intact lipid rafts, and its internalization occurs via dynamin-dependent endocytosis. The toxin is retrograde transported through the Golgi complex and the endoplasmic reticulum, and subsequently translocated into the nuclear compartment, where it exerts the toxic activity. Cellular intoxication induces DNA damage and activation of the DNA damage responses, which results in arrest of the target cells in the G1 and/or G2 phases of the cell cycle and activation of DNA repair mechanisms. Cells that fail to repair the damage will senesce or undergo apoptosis. This review will focus on the well-characterized aspects of the CDT biology and discuss the questions that still remain unanswered.
Keywords: DNA damage; DNA damage response; bacterial genotoxin; chancroid; colitis/hepatitis; cytolethal distending toxin; periodontitis; survival signals; toxin internalization; virulence factor.
Figures

References
-
- Nougayrede J.P., Homburg S., Taieb F., Boury M., Brzuszkiewicz E., Gottschalk G., Buchrieser C., Hacker J., Dobrindt U., Oswald E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–851. - PubMed
-
- Cortes-Bratti X., Frisan T., Thelestam M. The cytolethal distending toxins induce DNA damage and cell cycle arrest. Toxicon. 2001;39:1729–1736. - PubMed
-
- Thelestam M., Frisan T. Cytolethal distending toxins. In: Alouf J., Popoff M., editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. CPL Press; Newbury, UK: 2006. pp. 448–467.
-
- Nesic D., Hsu Y., Stebbins C.E. Assembly and function of a bacterial genotoxin. Nature. 2004;429:429–433. - PubMed
-
- Elwell C.A., Dreyfus L.A. DNAase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 2000;37:952–963. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

