Aggregatibacter actinomycetemcomitans leukotoxin: a powerful tool with capacity to cause imbalance in the host inflammatory response
- PMID: 22069708
- PMCID: PMC3202821
- DOI: 10.3390/toxins3030242
Aggregatibacter actinomycetemcomitans leukotoxin: a powerful tool with capacity to cause imbalance in the host inflammatory response
Abstract
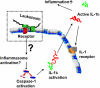
Aggregatibacter actinomycetemcomitans has been described as a member of the indigenous oral microbiota of humans, and is involved in the pathology of periodontitis and various non-oral infections. This bacterium selectively kills human leukocytes through expression of leukotoxin, a large pore-forming protein that belongs to the Repeat in Toxin (RTX) family. The specificity of the toxin is related to its prerequisite for a specific target cell receptor, LFA-1, which is solely expressed on leukocytes. The leukotoxin causes death of different leukocyte populations in a variety of ways. It activates a rapid release of lysosomal enzymes and MMPs from neutrophils and causes apoptosis in lymphocytes. In the monocytes/macrophages, the toxin activates caspase-1, a cysteine proteinase, which causes a proinflammatory response by the activation and secretion of IL-1β and IL-18. A specific clone (JP2) of A. actinomycetemcomitans with enhanced leukotoxin expression significantly correlates to disease onset in infected individuals. Taken together, the mechanisms by which this toxin kills leukocytes are closely related to the pathogenic mechanisms of inflammatory disorders, such as periodontitis. Therapeutic strategies targeting the cellular and molecular inflammatory host response in periodontal diseases might be a future treatment alternative.
Keywords: Aggregatibacter actinomycetemcomitans; leukotoxin; proinflammatory response; virulence mechanisms.
Figures



References
-
- Kinane D., Bouchard P. Group E of the European Workshop on Periodontology, Periodontal diseases and health: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008;35:333–337. - PubMed
-
- Darveau R.P. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8:481–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

