Mechanism of diphtheria toxin catalytic domain delivery to the eukaryotic cell cytosol and the cellular factors that directly participate in the process
- PMID: 22069710
- PMCID: PMC3202816
- DOI: 10.3390/toxins3030294
Mechanism of diphtheria toxin catalytic domain delivery to the eukaryotic cell cytosol and the cellular factors that directly participate in the process
Abstract
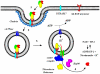
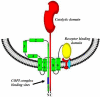
Research on diphtheria and anthrax toxins over the past three decades has culminated in a detailed understanding of their structure function relationships (e.g., catalytic (C), transmembrane (T), and receptor binding (R) domains), as well as the identification of their eukaryotic cell surface receptor, an understanding of the molecular events leading to the receptor-mediated internalization of the toxin into an endosomal compartment, and the pH triggered conformational changes required for pore formation in the vesicle membrane. Recently, a major research effort has been focused on the development of a detailed understanding of the molecular interactions between each of these toxins and eukaryotic cell factors that play an essential role in the efficient translocation of their respective catalytic domains through the trans-endosomal vesicle membrane pore and delivery into the cell cytosol. In this review, I shall focus on recent findings that have led to a more detailed understanding of the mechanism by which the diphtheria toxin catalytic domain is delivered to the eukaryotic cell cytosol. While much work remains, it is becoming increasingly clear that the entry process is facilitated by specific interactions with a number of cellular factors in an ordered sequential fashion. In addition, since diphtheria, anthrax lethal factor and anthrax edema factor all carry multiple coatomer I complex binding motifs and COPI complex has been shown to play an essential role in entry process, it is likely that the initial steps in catalytic domain entry of these divergent toxins follow a common mechanism.
Keywords: catalytic domain entry; coatomer complex I; diphtheria toxin.
Figures

Similar articles
-
Essential lysine residues within transmembrane helix 1 of diphtheria toxin facilitate COPI binding and catalytic domain entry.Mol Microbiol. 2010 May;76(4):1010-9. doi: 10.1111/j.1365-2958.2010.07159.x. Epub 2010 Apr 14. Mol Microbiol. 2010. PMID: 20398220 Free PMC article.
-
A conserved motif in transmembrane helix 1 of diphtheria toxin mediates catalytic domain delivery to the cytosol.Proc Natl Acad Sci U S A. 2005 Oct 25;102(43):15635-40. doi: 10.1073/pnas.0504937102. Epub 2005 Oct 17. Proc Natl Acad Sci U S A. 2005. PMID: 16230620 Free PMC article.
-
Trojan horse or proton force: finding the right partner(s) for toxin translocation.Neurotox Res. 2006 Apr;9(2-3):63-71. doi: 10.1007/BF03033924. Neurotox Res. 2006. PMID: 16785102 Review.
-
COPI coatomer complex proteins facilitate the translocation of anthrax lethal factor across vesicular membranes in vitro.Proc Natl Acad Sci U S A. 2008 Apr 1;105(13):5254-9. doi: 10.1073/pnas.0710100105. Epub 2008 Mar 20. Proc Natl Acad Sci U S A. 2008. PMID: 18356299 Free PMC article.
-
pH-triggered conformational switching along the membrane insertion pathway of the diphtheria toxin T-domain.Toxins (Basel). 2013 Aug 6;5(8):1362-80. doi: 10.3390/toxins5081362. Toxins (Basel). 2013. PMID: 23925141 Free PMC article. Review.
Cited by
-
AB Toxins as High-Affinity Ligands for Cell Targeting in Cancer Therapy.Int J Mol Sci. 2023 Jul 7;24(13):11227. doi: 10.3390/ijms241311227. Int J Mol Sci. 2023. PMID: 37446406 Free PMC article. Review.
-
Diphtheria toxin-based bivalent human IL-2 fusion toxin with improved efficacy for targeting human CD25(+) cells.J Immunol Methods. 2014 Mar;405:57-66. doi: 10.1016/j.jim.2014.01.008. Epub 2014 Jan 24. J Immunol Methods. 2014. PMID: 24462799 Free PMC article.
-
Novel Toxin-Antitoxin Module SlvT-SlvA Regulates Megaplasmid Stability and Incites Solvent Tolerance in Pseudomonas putida S12.Appl Environ Microbiol. 2020 Jun 17;86(13):e00686-20. doi: 10.1128/AEM.00686-20. Print 2020 Jun 17. Appl Environ Microbiol. 2020. PMID: 32358012 Free PMC article.
-
Subcellular Trafficking of the Papillomavirus Genome during Initial Infection: The Remarkable Abilities of Minor Capsid Protein L2.Viruses. 2017 Dec 3;9(12):370. doi: 10.3390/v9120370. Viruses. 2017. PMID: 29207511 Free PMC article. Review.
-
Dmp1 Promoter-Driven Diphtheria Toxin Receptor Transgene Expression Directs Unforeseen Effects in Multiple Tissues.Int J Mol Sci. 2016 Dec 26;18(1):29. doi: 10.3390/ijms18010029. Int J Mol Sci. 2016. PMID: 28035954 Free PMC article.
References
-
- Pappenheimer A.M., Jr. Diphtheria toxin. Ann. Rev. Biochem. 1977;46:69–94. - PubMed
-
- Kaczorek M., Delpeyroux F., Chenciner N., Streeck R.E., Murphy J.R., Boquet P., Tiollais P. Nucleotide sequence and expression of the diphtheria tox228 gene in Escherichia coli. Science. 1983;221:855–858. - PubMed
-
- Collier R.J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J. Biol. Chem. 1971;246:1496–1503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

