Use of ribosome-inactivating proteins from Sambucus for the construction of immunotoxins and conjugates for cancer therapy
- PMID: 22069717
- PMCID: PMC3202832
- DOI: 10.3390/toxins3050420
Use of ribosome-inactivating proteins from Sambucus for the construction of immunotoxins and conjugates for cancer therapy
Abstract
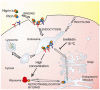
The type 2 ribosome-inactivating proteins (RIPs) isolated from some species belonging to the Sambucus genus, have the characteristic that although being even more active than ricin inhibiting protein synthesis in cell-free extracts, they lack the high toxicity of ricin and related type 2 RIPs to intact cells and animals. This is due to the fact that after internalization, they follow a different intracellular pathway that does not allow them to reach the cytosolic ribosomes. The lack of toxicity of type 2 RIPs from Sambucus make them good candidates as toxic moieties in the construction of immunotoxins and conjugates directed against specific targets. Up to now they have been conjugated with either transferrin or anti-CD105 to target either transferrin receptor- or endoglin-overexpressing cells, respectively.
Keywords: ribosome-inactivating proteins; Sambucus; endoglin (CD105); immunotoxin; transferrin.
Figures
References
-
- Barbieri L., Battelli M.G., Stirpe F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta. 1993;1154:237–282. - PubMed
-
- Girbes T., Ferreras J.M., Arias F.J., Stirpe F. Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini Rev. Med. Chem. 2004;4:461–476. - PubMed
-
- Stirpe F. Ribosome-inactivating proteins. Toxicon. 2004;44:371–383. - PubMed
-
- Hartley M.R., Lord J.M. Cytotoxic ribosome-inactivating lectins from plants. Biochim. Biophys. Acta. 2004;1701:1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

