All-time releases of mercury to the atmosphere from human activities
- PMID: 22070723
- PMCID: PMC3246392
- DOI: 10.1021/es202765m
All-time releases of mercury to the atmosphere from human activities
Abstract
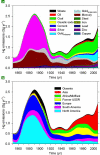
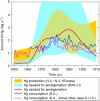
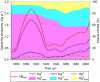
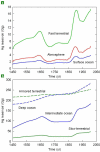
Understanding the biogeochemical cycling of mercury is critical for explaining the presence of mercury in remote regions of the world, such as the Arctic and the Himalayas, as well as local concentrations. While we have good knowledge of present-day fluxes of mercury to the atmosphere, we have little knowledge of what emission levels were like in the past. Here we develop a trend of anthropogenic emissions of mercury to the atmosphere from 1850 to 2008-for which relatively complete data are available-and supplement that trend with an estimate of anthropogenic emissions prior to 1850. Global mercury emissions peaked in 1890 at 2600 Mg yr(-1), fell to 700-800 Mg yr(-1) in the interwar years, then rose steadily after 1950 to present-day levels of 2000 Mg yr(-1). Our estimate for total mercury emissions from human activities over all time is 350 Gg, of which 39% was emitted before 1850 and 61% after 1850. Using an eight-compartment global box-model of mercury biogeochemical cycling, we show that these emission trends successfully reproduce present-day atmospheric enrichment in mercury.
Figures

References
-
- Fitzgerald WF, Engstrom DR, Mason RP, Nater EA. The case for atmospheric mercury contamination in remote areas. Environ. Sci. Technol. 1998;32:1–7.
-
- Durnford D, Dastoor A, Figueras-Nieto D, Ryjkov A. Long range transport of mercury to the Arctic and across Canada. Atmos. Chem. Phys. 2010;10:6063–6086.
-
- Temme C, Einax JW, Ebinghaus R, Schroeder WH. Measurements of atmospheric mercury species at a coastal site in the Antarctic and over the South Atlantic Ocean during polar summer. Environ. Sci. Technol. 2003;37:22–31. - PubMed
-
- Loewen M, Kang S, Armstrong D, Zhang Q, Tomy G, Wang F. Atmospheric transport of mercury to the Tibetan Plateau. Environ. Sci. Technol. 2007;41:7632–7638. - PubMed
-
- Pirrone N, Mason R, editors. Mercury Fate and Transport in the Global Atmosphere. Springer; New York: 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

