Haploinsufficiency of the genes encoding the tumor suppressor Pten predisposes zebrafish to hemangiosarcoma
- PMID: 22071262
- PMCID: PMC3291645
- DOI: 10.1242/dmm.008326
Haploinsufficiency of the genes encoding the tumor suppressor Pten predisposes zebrafish to hemangiosarcoma
Abstract
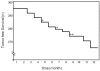
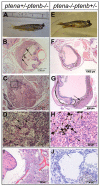
PTEN is an essential tumor suppressor that antagonizes Akt/PKB signaling. The zebrafish genome encodes two Pten genes, ptena and ptenb. Here, we report that zebrafish mutants that retain a single wild-type copy of ptena or ptenb (ptena(+/-)ptenb(-/-) or ptena(-/-)ptenb(+/-)) are viable and fertile. ptena(+/-)ptenb(-/-) fish develop tumors at a relatively high incidence (10.2%) and most tumors developed close to the eye (26/30). Histopathologically, the tumor masses were associated with the retrobulbar vascular network and diagnosed as hemangiosarcomas. A single tumor was identified in 42 ptena(-/-)ptenb(+/-) fish and was also diagnosed as hemangiosarcoma. Immunohistochemistry indicated that the tumor cells in ptena(+/-)ptenb(-/-) and ptena(-/-)ptenb(+/-) fish proliferated rapidly and were of endothelial origin. Akt/PKB signaling was activated in the tumors, whereas Ptena was still detected in tumor tissue from ptena(+/-)ptenb(-/-) zebrafish. We conclude that haploinsufficiency of the genes encoding Pten predisposes to hemangiosarcoma in zebrafish.
Figures


References
-
- Ali I. U., Schriml L. M., Dean M. (1999). Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J. Natl. Cancer Inst. 91, 1922–1932 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

