Two types of luminescence blinking revealed by spectroelectrochemistry of single quantum dots
- PMID: 22071764
- PMCID: PMC3390028
- DOI: 10.1038/nature10569
Two types of luminescence blinking revealed by spectroelectrochemistry of single quantum dots
Abstract
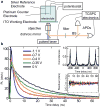
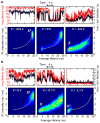
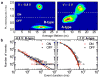
Photoluminescence blinking--random switching between states of high (ON) and low (OFF) emissivities--is a universal property of molecular emitters found in dyes, polymers, biological molecules and artificial nanostructures such as nanocrystal quantum dots, carbon nanotubes and nanowires. For the past 15 years, colloidal nanocrystals have been used as a model system to study this phenomenon. The occurrence of OFF periods in nanocrystal emission has been commonly attributed to the presence of an additional charge, which leads to photoluminescence quenching by non-radiative recombination (the Auger mechanism). However, this 'charging' model was recently challenged in several reports. Here we report time-resolved photoluminescence studies of individual nanocrystal quantum dots performed while electrochemically controlling the degree of their charging, with the goal of clarifying the role of charging in blinking. We find that two distinct types of blinking are possible: conventional (A-type) blinking due to charging and discharging of the nanocrystal core, in which lower photoluminescence intensities correlate with shorter photoluminescence lifetimes; and a second sort (B-type), in which large changes in the emission intensity are not accompanied by significant changes in emission dynamics. We attribute B-type blinking to charge fluctuations in the electron-accepting surface sites. When unoccupied, these sites intercept 'hot' electrons before they relax into emitting core states. Both blinking mechanisms can be electrochemically controlled and completely suppressed by application of an appropriate potential.
Figures


References
-
- Hoogenboom JP, Hernando J, van Dijk EMHP, van Hulst NF, García-Parajó MF. Power-law blinking in the fluorescence of single organic molecules. ChemPhysChem. 2007;8:823–833. - PubMed
-
- Bout DAV, et al. Discrete intensity jumps and intramolecular electronic energy transfer in the spectroscopy of single conjugated polymer molecules. Science. 1997;277:1074–1077.
-
- Riley EA, Bingham C, Bott ED, Kahr B, Reid PJ. Two mechanisms for fluorescence intermittency of single violamine R molecules. Phys Chem Chem Phys. 2011;13:1879–1887. - PubMed
-
- Frantsuzov P, Kuno M, Janko B, Marcus RA. Universal emission intermittency in quantum dots, nanorods and nanowires. Nat Phys. 2008;4:519–522.
-
- Nirmal M, et al. Fluorescence intermittency in single cadmium selenide nanocrystals. Nature. 1996;383:802–804.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

