Modulation of renal superoxide dismutase by telmisartan therapy in C57BL/6-Ins2(Akita) diabetic mice
- PMID: 22072110
- PMCID: PMC3273720
- DOI: 10.1038/hr.2011.176
Modulation of renal superoxide dismutase by telmisartan therapy in C57BL/6-Ins2(Akita) diabetic mice
Abstract
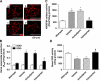
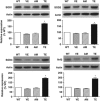
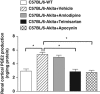
Renal superoxide excess, which is induced by an imbalance of the superoxide-producing enzyme NAD(P)H oxidase and the superoxide-scavenging enzyme superoxide dismutase (SOD) under hyperglycemia, increases oxidative stress and contributes to the development of diabetic nephropathy. In this study, we treated non-obese and hypoinsulinemic C57BL/6-Ins2(Akita) (C57BL/6-Akita) diabetic mice with telmisartan (5 mg kg(-1) per day), an angiotensin II type 1 receptor blocker, or amlodipine (5 mg kg(-1) per day), a calcium channel blocker, for 4 weeks and compared the effects of these two anti-hypertensive drugs on renal NAD(P)H oxidase, SOD and transcription factor Nrf2 (NF-E2-related factor 2), which is known to upregulate several antioxidant enzymes including SOD. Vehicle-treated C57BL/6-Akita mice exhibited higher renal NAD(P)H oxidase and lower renal SOD activity with increased levels of renal superoxide than the C57BL/6-wild-type non-diabetic mice. Interestingly, telmisartan treatment not only reduced NAD(P)H oxidase activity but also enhanced SOD activity in C57BL/6-Akita mouse kidneys, leading to a reduction of renal superoxide levels. Furthermore, telmisartan-treated C57BL/6-Akita mice increased the renal protein expression of SOD and Nrf2. In parallel with the reduction of renal superoxide levels, a reduction of urinary albumin levels and a normalization of elevated glomerular filtration rate were observed in telmisartan-treated C57BL/6-Akita mice. In contrast, treatment with amlodipine failed to modulate renal NAD(P)H oxidase, SOD and Nrf2. Finally, treatment of C57BL/6-Akita mice with apocynin, an NAD(P)H oxidase inhibitor, also increased the renal protein expression of SOD and Nrf2. Collectively, our data suggest that NAD(P)H oxidase negatively regulates renal SOD, possibly by downregulation of Nrf2, and that telmisartan could upregulate renal SOD by the suppression of NAD(P)H oxidase and subsequent upregulation of Nrf2, leading to the amelioration of renal oxidative stress and diabetic renal changes.
Figures


Similar articles
-
SOD1, but not SOD3, deficiency accelerates diabetic renal injury in C57BL/6-Ins2(Akita) diabetic mice.Metabolism. 2012 Dec;61(12):1714-24. doi: 10.1016/j.metabol.2012.05.005. Epub 2012 May 25. Metabolism. 2012. PMID: 22632894 Free PMC article.
-
Renoprotective activity of telmisartan versus pioglitazone on ischemia/reperfusion induced renal damage in diabetic rats.Eur Rev Med Pharmacol Sci. 2012 May;16(5):600-9. Eur Rev Med Pharmacol Sci. 2012. PMID: 22774400
-
SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice.Am J Physiol Renal Physiol. 2014 Jan;306(2):F194-204. doi: 10.1152/ajprenal.00520.2013. Epub 2013 Nov 13. Am J Physiol Renal Physiol. 2014. PMID: 24226524 Free PMC article.
-
Telmisartan in incipient and overt diabetic renal disease.J Nephrol. 2011 May-Jun;24(3):263-73. doi: 10.5301/JN.2011.6416. J Nephrol. 2011. PMID: 21374585 Review.
-
Telmisartan in the management of diabetic nephropathy: a contemporary view.Curr Diabetes Rev. 2012 May;8(3):183-90. doi: 10.2174/157339912800563972. Curr Diabetes Rev. 2012. PMID: 22429010 Review.
Cited by
-
Fimasartan, a Novel Angiotensin-Receptor Blocker, Protects against Renal Inflammation and Fibrosis in Mice with Unilateral Ureteral Obstruction: the Possible Role of Nrf2.Int J Med Sci. 2015 Oct 21;12(11):891-904. doi: 10.7150/ijms.13187. eCollection 2015. Int J Med Sci. 2015. PMID: 26640409 Free PMC article.
-
Effects of Single and Combined Losartan and Tempol Treatments on Oxidative Stress, Kidney Structure and Function in Spontaneously Hypertensive Rats with Early Course of Proteinuric Nephropathy.PLoS One. 2016 Aug 25;11(8):e0161706. doi: 10.1371/journal.pone.0161706. eCollection 2016. PLoS One. 2016. PMID: 27560781 Free PMC article.
-
Deletion of p47phox attenuates the progression of diabetic nephropathy and reduces the severity of diabetes in the Akita mouse.Diabetologia. 2012 Sep;55(9):2522-32. doi: 10.1007/s00125-012-2586-1. Epub 2012 Jun 1. Diabetologia. 2012. PMID: 22653270
-
Regression of Renal Disease by Angiotensin II Antagonism Is Caused by Regeneration of Kidney Vasculature.J Am Soc Nephrol. 2016 Mar;27(3):699-705. doi: 10.1681/ASN.2014100971. Epub 2015 Jun 26. J Am Soc Nephrol. 2016. PMID: 26116358 Free PMC article.
-
Effect of Telmisartan in the Oxidative Stress Components Induced by Ischemia Reperfusion in Rats.Oxid Med Cell Longev. 2019 Jul 2;2019:1302985. doi: 10.1155/2019/1302985. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31354899 Free PMC article.
References
-
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. - PubMed
-
- Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP, Brownlee M, Bierhaus A, Cooper ME, Forbes JM. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–752. - PMC - PubMed
-
- Ghosh S, Khazaei M, Moien-Afshari F, Ang LS, Granville DJ, Verchere CB, Dunn SR, McCue P, Mizisin A, Sharma K, Laher I. Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. Am J Physiol Renal Physiol. 2009;296:F700–F708. - PMC - PubMed
-
- Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57:460–469. - PubMed
-
- Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
