Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans
- PMID: 22072556
- PMCID: PMC3286218
- DOI: 10.1182/blood-2011-07-364414
Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans
Abstract
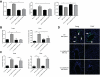
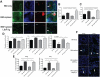
Chronic GVHD (cGVHD) poses a significant risk for HSCT patients. Preclinical development of new therapeutic modalities has been hindered by models with pathologic findings that may not simulate the development of human cGVHD. Previously, we have demonstrated that cGVHD induced by allogeneic HSCT after a conditioning regimen of cyclophosphamide and total-body radiation results in pulmonary dysfunction and airway obliteration, which leads to bronchiolitis obliterans (BO), which is pathognomonic for cGVHD of the lung. We now report cGVHD manifestations in a wide spectrum of target organs, including those with mucosal surfaces. Fibrosis was demonstrated in the lung and liver and was associated with CD4(+) T cells and B220(+) B-cell infiltration and alloantibody deposition. Donor bone marrow obtained from mice incapable of secreting IgG alloantibody resulted in less BO and cGVHD. Robust germinal center reactions were present at the time of cGVHD disease initiation. Blockade of germinal center formation with a lymphotoxin-receptor-immunoglobulin fusion protein suppressed cGVHD and BO. We conclude that cGVHD is caused in part by alloantibody secretion, which is associated with fibrosis and cGVHD manifestations including BO, and that treatment with a lymphotoxin-β receptor-immunoglobulin fusion protein could be beneficial for cGVHD prevention and therapy.
Figures
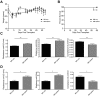
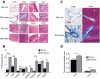

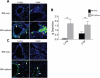
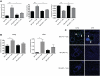
Comment in
-
Exciting new murine model of cGVHD.Blood. 2012 Feb 9;119(6):1331-2. doi: 10.1182/blood-2011-12-393496. Blood. 2012. PMID: 22323409
References
-
- Baird K, Pavletic SZ. Chronic graft versus host disease. Curr Opin Hematol. 2006;13(6):426–435. - PubMed
-
- Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease, I: diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. - PubMed
-
- Socie G, Ritz J, Martin PJ. Current challenges in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(1 suppl):S146–S151. - PubMed
-
- Kapur R, Ebeling S, Hagenbeek A. B-cell involvement in chronic graft-versus-host disease. Haematologica. 2008;93(11):1702–1711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

