Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone), isolated from Plumbago zeylanica, inhibits ultraviolet radiation-induced development of squamous cell carcinomas
- PMID: 22072620
- PMCID: PMC3276331
- DOI: 10.1093/carcin/bgr249
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone), isolated from Plumbago zeylanica, inhibits ultraviolet radiation-induced development of squamous cell carcinomas
Abstract
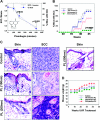
Plumbagin (PL) (5-hydroxy-2-methyl-1,4-napthoquinone), a medicinal plant-derived naphthoquinone, was isolated from the roots of the Plumbago zeylanica L. (also known as Chitrak). The roots of P. zeylanica L. have been used in Indian medicine for >2500 years as an anti-atherogenic, cardiotonic, hepatoprotective and neuroprotective agent. We present here that topical application of non-toxic doses (100-500 nmol) of PL to skin elicits dose-dependent inhibition of ultraviolet radiation (UVR)-induced development of squamous cell carcinomas (SCC). In this experiment, FVB/N mice were exposed to UVR (2 kJ/m(2)) three times weekly from a bank of six Kodacel-filtered FS40 sunlamps (∼ 60% UVB and 40% UVA). Carcinoma incidence in mice treated with vehicle, 100, 200 or 500 nmol PL, at 44 weeks post-UVR, were 86, 80 (P = 0.67), 53 (P = 0.12) and 7% (P = 0.0075), respectively. Both vehicle and PL-treated mice gained weight and did not exhibit any signs of toxicity during the entire period of the experiment. Molecular mechanisms associated with inhibition of UVR-induced development of SCC involved induction of apoptosis and inhibition of cell proliferation. Specific findings are that PL treatment (i) inhibited UVR-induced DNA binding of activating protein-1, nuclear factor-kappaB, Stat3 transcription factors and Stat3-regulated molecules (cdc25A and Survivin); (ii) inhibited protein levels of pERK1/2, PI3K85, pAKTSer473, Bcl(2), BclxL, proliferating cell nuclear antigen and cell cycle inhibitory proteins p27 and p21 and (iii) increased UVR-induced Fas-associated death domain expression, poly (ADP-ribose) polymerase protein cleavage and Bax/Bcl(2) ratio. Taken together, our findings suggest that PL may be a novel agent for the prevention of skin cancer.
Figures



Similar articles
-
Plumbagin inhibits prostate cancer development in TRAMP mice via targeting PKCε, Stat3 and neuroendocrine markers.Carcinogenesis. 2012 Dec;33(12):2586-92. doi: 10.1093/carcin/bgs291. Epub 2012 Sep 13. Carcinogenesis. 2012. PMID: 22976928 Free PMC article.
-
PKCepsilon overexpression, irrespective of genetic background, sensitizes skin to UVR-induced development of squamous-cell carcinomas.J Invest Dermatol. 2010 Jan;130(1):270-7. doi: 10.1038/jid.2009.212. J Invest Dermatol. 2010. PMID: 19626035 Free PMC article.
-
Plumbagin, a medicinal plant (Plumbago zeylanica)-derived 1,4-naphthoquinone, inhibits growth and metastasis of human prostate cancer PC-3M-luciferase cells in an orthotopic xenograft mouse model.Mol Oncol. 2013 Jun;7(3):428-39. doi: 10.1016/j.molonc.2012.12.001. Epub 2012 Dec 14. Mol Oncol. 2013. PMID: 23273564 Free PMC article.
-
Sunscreens.Adv Exp Med Biol. 2014;810:429-63. doi: 10.1007/978-1-4939-0437-2_25. Adv Exp Med Biol. 2014. PMID: 25207381 Review.
-
UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer.Adv Exp Med Biol. 2017;996:27-40. doi: 10.1007/978-3-319-56017-5_3. Adv Exp Med Biol. 2017. PMID: 29124688 Review.
Cited by
-
Plumbagin Inhibits Prostate Carcinogenesis in Intact and Castrated PTEN Knockout Mice via Targeting PKCε, Stat3, and Epithelial-to-Mesenchymal Transition Markers.Cancer Prev Res (Phila). 2015 May;8(5):375-86. doi: 10.1158/1940-6207.CAPR-14-0231. Epub 2015 Jan 27. Cancer Prev Res (Phila). 2015. PMID: 25627799 Free PMC article.
-
Nanoparticle-Based Celecoxib and Plumbagin for the Synergistic Treatment of Melanoma.Mol Cancer Ther. 2017 Mar;16(3):440-452. doi: 10.1158/1535-7163.MCT-16-0285. Epub 2016 Dec 21. Mol Cancer Ther. 2017. PMID: 28003325 Free PMC article.
-
Plumbagin-induced oxidative stress leads to inhibition of Na+/K+-ATPase (NKA) in canine cancer cells.Sci Rep. 2019 Aug 7;9(1):11471. doi: 10.1038/s41598-019-47261-x. Sci Rep. 2019. PMID: 31391478 Free PMC article.
-
Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy.Polymers (Basel). 2021 Mar 17;13(6):923. doi: 10.3390/polym13060923. Polymers (Basel). 2021. PMID: 33802819 Free PMC article.
-
Evaluation of the inhibition potential of plumbagin against cytochrome P450 using LC-MS/MS and cocktail approach.Sci Rep. 2016 Jun 22;6:28482. doi: 10.1038/srep28482. Sci Rep. 2016. PMID: 27329697 Free PMC article.
References
-
- Jemal A, et al. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59:225–249. - PubMed
-
- Armstrong BK, et al. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B. 2001;63:8–18. - PubMed
-
- de Gruijl FR. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol. Appl. Skin Physiol. 2002;15:316–320. - PubMed
-
- Nguyen TH, et al. Nonmelanoma skin cancer. Curr. Treat. Options Oncol. 2002;3:193–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

