Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones
- PMID: 22072663
- PMCID: PMC3249406
- DOI: 10.1523/JNEUROSCI.4175-11.2011
Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones
Abstract
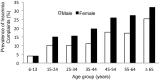
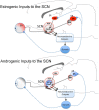
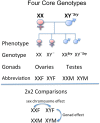
While much is known about the mechanisms that underlie sleep and circadian rhythms, the investigation into sex differences and gonadal steroid modulation of sleep and biological rhythms is in its infancy. There is a growing recognition of sex disparities in sleep and rhythm disorders. Understanding how neuroendocrine mediators and sex differences influence sleep and biological rhythms is central to advancing our understanding of sleep-related disorders. While it is known that ovarian steroids affect circadian rhythms in rodents, the role of androgen is less understood. Surprising findings that androgens, acting via androgen receptors in the master "circadian clock" within the suprachiasmatic nucleus, modulate photic effects on activity in males point to novel mechanisms of circadian control. Work in aromatase-deficient mice suggests that some sex differences in photic responsiveness are independent of gonadal hormone effects during development. In parallel, aspects of sex differences in sleep are also reported to be independent of gonadal steroids and may involve sex chromosome complement. This a summary of recent work illustrating how sex differences and gonadal hormones influence sleep and circadian rhythms that was presented at a Mini-Symposium at the 2011 annual meeting of the Society for Neuroscience.
Figures
References
-
- Abizaid A, Mezei G, Thanarajasingam G, Horvath TL. Estrogen enhances light-induced activation of dorsal raphe serotonergic neurons. Eur J Neurosci. 2005;21:1536–1546. - PubMed
-
- Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76:636–652. - PubMed
-
- Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241:R62–R66. - PubMed
-
- Albers HE, Gerall AA, Axelson JF. Effect of reproductive state on circadian periodicity in the rat. Physiol Behav. 1981;26:21–25. - PubMed
-
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 NS034194/NS/NINDS NIH HHS/United States
- R01 MH075045/MH/NIMH NIH HHS/United States
- R21 HL088088/HL/NHLBI NIH HHS/United States
- R01 NS078410/NS/NINDS NIH HHS/United States
- R01 HL085037/HL/NHLBI NIH HHS/United States
- NS060659/NS/NINDS NIH HHS/United States
- R01 NS037919/NS/NINDS NIH HHS/United States
- MH075045/MH/NIMH NIH HHS/United States
- NS37919/NS/NINDS NIH HHS/United States
- R01 HL103688/HL/NHLBI NIH HHS/United States
- HL088088/HL/NHLBI NIH HHS/United States
- NS34194/NS/NINDS NIH HHS/United States
- L60 MD003227/MD/NIMHD NIH HHS/United States
- HL103688/HL/NHLBI NIH HHS/United States
- HL085037/HL/NHLBI NIH HHS/United States
- U54 NS060659/NS/NINDS NIH HHS/United States
- MOP-67085/CAPMC/ CIHR/Canada
LinkOut - more resources
Full Text Sources
