Dendritic spine dynamics regulate the long-term stability of synaptic plasticity
- PMID: 22072667
- PMCID: PMC6633231
- DOI: 10.1523/JNEUROSCI.2520-11.2011
Dendritic spine dynamics regulate the long-term stability of synaptic plasticity
Abstract
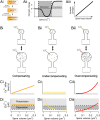
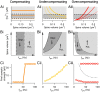
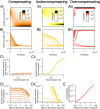
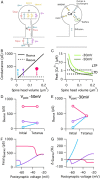
Long-term synaptic plasticity requires postsynaptic influx of Ca²⁺ and is accompanied by changes in dendritic spine size. Unless Ca²⁺ influx mechanisms and spine volume scale proportionally, changes in spine size will modify spine Ca²⁺ concentrations during subsequent synaptic activation. We show that the relationship between Ca²⁺ influx and spine volume is a fundamental determinant of synaptic stability. If Ca²⁺ influx is undercompensated for increases in spine size, then strong synapses are stabilized and synaptic strength distributions have a single peak. In contrast, overcompensation of Ca²⁺ influx leads to binary, persistent synaptic strengths with double-peaked distributions. Biophysical simulations predict that CA1 pyramidal neuron spines are undercompensating. This unifies experimental findings that weak synapses are more plastic than strong synapses, that synaptic strengths are unimodally distributed, and that potentiation saturates for a given stimulus strength. We conclude that structural plasticity provides a simple, local, and general mechanism that allows dendritic spines to foster both rapid memory formation and persistent memory storage.
Figures



Similar articles
-
Intracellular calcium stores mediate metaplasticity at hippocampal dendritic spines.J Physiol. 2019 Jul;597(13):3473-3502. doi: 10.1113/JP277726. Epub 2019 Jun 2. J Physiol. 2019. PMID: 31099020 Free PMC article.
-
Chemically induced long-term potentiation increases the number of perforated and complex postsynaptic densities but does not alter dendritic spine volume in CA1 of adult mouse hippocampal slices.Eur J Neurosci. 2005 Jun;21(12):3368-78. doi: 10.1111/j.1460-9568.2005.04174.x. Eur J Neurosci. 2005. PMID: 16026474
-
Do thin spines learn to be mushroom spines that remember?Curr Opin Neurobiol. 2007 Jun;17(3):381-6. doi: 10.1016/j.conb.2007.04.009. Epub 2007 May 10. Curr Opin Neurobiol. 2007. PMID: 17498943 Review.
-
Calcium control of triphasic hippocampal STDP.J Comput Neurosci. 2012 Dec;33(3):495-514. doi: 10.1007/s10827-012-0397-5. Epub 2012 May 19. J Comput Neurosci. 2012. PMID: 22610510
-
Spine dynamics and synapse remodeling during LTP and memory processes.Prog Brain Res. 2008;169:199-207. doi: 10.1016/S0079-6123(07)00011-8. Prog Brain Res. 2008. PMID: 18394475 Review.
Cited by
-
Fmr1-KO mice failure to detect object novelty associates with a post-test decrease of structural and synaptic plasticity upstream of the hippocampus.Sci Rep. 2023 Jan 14;13(1):755. doi: 10.1038/s41598-023-27991-9. Sci Rep. 2023. PMID: 36641518 Free PMC article.
-
Intracellular calcium stores mediate metaplasticity at hippocampal dendritic spines.J Physiol. 2019 Jul;597(13):3473-3502. doi: 10.1113/JP277726. Epub 2019 Jun 2. J Physiol. 2019. PMID: 31099020 Free PMC article.
-
Pannexin 1 Regulates Dendritic Protrusion Dynamics in Immature Cortical Neurons.eNeuro. 2020 Aug 26;7(4):ENEURO.0079-20.2020. doi: 10.1523/ENEURO.0079-20.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32737184 Free PMC article.
-
Memory retention in pyramidal neurons: a unified model of energy-based homo and heterosynaptic plasticity with homeostasis.Cogn Neurodyn. 2021 Aug;15(4):675-692. doi: 10.1007/s11571-020-09652-z. Epub 2020 Nov 17. Cogn Neurodyn. 2021. PMID: 34367368 Free PMC article.
-
How inhibitory and excitatory inputs gate output of the inferior olive.Elife. 2023 Aug 1;12:e83239. doi: 10.7554/eLife.83239. Elife. 2023. PMID: 37526175 Free PMC article.
References
-
- Artola A, Bröcher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. - PubMed
-
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. - PubMed
-
- Barbour B, Brunel N, Hakim V, Nadal JP. What can we learn from synaptic weight distributions? Trends Neurosci. 2007;30:622–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
