Two distinct modes of forebrain circuit dynamics underlie temporal patterning in the vocalizations of young songbirds
- PMID: 22072687
- PMCID: PMC3241969
- DOI: 10.1523/JNEUROSCI.3009-11.2011
Two distinct modes of forebrain circuit dynamics underlie temporal patterning in the vocalizations of young songbirds
Abstract
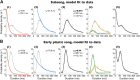
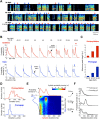
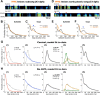
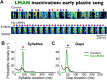
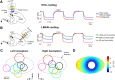
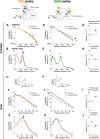
Accurate timing is a critical aspect of motor control, yet the temporal structure of many mature behaviors emerges during learning from highly variable exploratory actions. How does a developing brain acquire the precise control of timing in behavioral sequences? To investigate the development of timing, we analyzed the songs of young juvenile zebra finches. These highly variable vocalizations, akin to human babbling, gradually develop into temporally stereotyped adult songs. We find that the durations of syllables and silences in juvenile singing are formed by a mixture of two distinct modes of timing: a random mode producing broadly distributed durations early in development, and a stereotyped mode underlying the gradual emergence of stereotyped durations. Using lesions, inactivations, and localized brain cooling, we investigated the roles of neural dynamics within two premotor cortical areas in the production of these temporal modes. We find that LMAN (lateral magnocellular nucleus of the nidopallium) is required specifically for the generation of the random mode of timing and that mild cooling of LMAN causes an increase in the durations produced by this mode. On the contrary, HVC (used as a proper name) is required specifically for producing the stereotyped mode of timing, and its cooling causes a slowing of all stereotyped components. These results show that two neural pathways contribute to the timing of juvenile songs and suggest an interesting organization in the forebrain, whereby different brain areas are specialized for the production of distinct forms of neural dynamics.
Figures




References
-
- Abeles M. Corticonics. Cambridge, UK: Cambridge UP; 1991.
-
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
