Tonic nanomolar dopamine enables an activity-dependent phase recovery mechanism that persistently alters the maximal conductance of the hyperpolarization-activated current in a rhythmically active neuron
- PMID: 22072689
- PMCID: PMC3758573
- DOI: 10.1523/JNEUROSCI.3770-11.2011
Tonic nanomolar dopamine enables an activity-dependent phase recovery mechanism that persistently alters the maximal conductance of the hyperpolarization-activated current in a rhythmically active neuron
Abstract
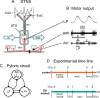
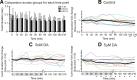
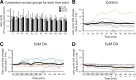
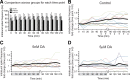
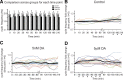
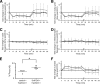
The phases at which network neurons fire in rhythmic motor outputs are critically important for the proper generation of motor behaviors. The pyloric network in the crustacean stomatogastric ganglion generates a rhythmic motor output wherein neuronal phase relationships are remarkably invariant across individuals and throughout lifetimes. The mechanisms for maintaining these robust phase relationships over the long-term are not well described. Here we show that tonic nanomolar dopamine (DA) acts at type 1 DA receptors (D1Rs) to enable an activity-dependent mechanism that can contribute to phase maintenance in the lateral pyloric (LP) neuron. The LP displays continuous rhythmic bursting. The activity-dependent mechanism was triggered by a prolonged decrease in LP burst duration, and it generated a persistent increase in the maximal conductance (G(max)) of the LP hyperpolarization-activated current (I(h)), but only in the presence of steady-state DA. Interestingly, micromolar DA produces an LP phase advance accompanied by a decrease in LP burst duration that abolishes normal LP network function. During a 1 h application of micromolar DA, LP phase recovered over tens of minutes because, the activity-dependent mechanism enabled by steady-state DA was triggered by the micromolar DA-induced decrease in LP burst duration. Presumably, this mechanism restored normal LP network function. These data suggest steady-state DA may enable homeostatic mechanisms that maintain motor network output during protracted neuromodulation. This DA-enabled, activity-dependent mechanism to preserve phase may be broadly relevant, as diminished dopaminergic tone has recently been shown to reduce I(h) in rhythmically active neurons in the mammalian brain.
Figures




Similar articles
-
Cell specific dopamine modulation of the transient potassium current in the pyloric network by the canonical D1 receptor signal transduction cascade.J Neurophysiol. 2010 Aug;104(2):873-84. doi: 10.1152/jn.00195.2010. Epub 2010 Jun 2. J Neurophysiol. 2010. PMID: 20519576 Free PMC article.
-
Tonic 5nM DA stabilizes neuronal output by enabling bidirectional activity-dependent regulation of the hyperpolarization activated current via PKA and calcineurin.PLoS One. 2015 Feb 18;10(2):e0117965. doi: 10.1371/journal.pone.0117965. eCollection 2015. PLoS One. 2015. PMID: 25692473 Free PMC article.
-
Dopamine modulation of two delayed rectifier potassium currents in a small neural network.J Neurophysiol. 2005 Oct;94(4):2888-900. doi: 10.1152/jn.00434.2005. Epub 2005 Jul 13. J Neurophysiol. 2005. PMID: 16014791
-
Dopamine modulation of two subthreshold currents produces phase shifts in activity of an identified motoneuron.J Neurophysiol. 1995 Oct;74(4):1404-20. doi: 10.1152/jn.1995.74.4.1404. J Neurophysiol. 1995. PMID: 8989381
-
Neuromodulatory inputs maintain expression of a lobster motor pattern-generating network in a modulation-dependent state: evidence from long-term decentralization in vitro.J Neurosci. 1998 Mar 15;18(6):2212-25. doi: 10.1523/JNEUROSCI.18-06-02212.1998. J Neurosci. 1998. PMID: 9482805 Free PMC article. Review.
Cited by
-
Homeostatic plasticity of excitability in crustacean central pattern generator networks.Curr Opin Neurobiol. 2017 Apr;43:7-14. doi: 10.1016/j.conb.2016.09.015. Epub 2016 Oct 6. Curr Opin Neurobiol. 2017. PMID: 27721084 Free PMC article. Review.
-
Neuromodulation of neuronal circuits: back to the future.Neuron. 2012 Oct 4;76(1):1-11. doi: 10.1016/j.neuron.2012.09.010. Neuron. 2012. PMID: 23040802 Free PMC article. Review.
-
A codimension-2 bifurcation controlling endogenous bursting activity and pulse-triggered responses of a neuron model.PLoS One. 2014 Jan 31;9(1):e85451. doi: 10.1371/journal.pone.0085451. eCollection 2014. PLoS One. 2014. PMID: 24497927 Free PMC article.
-
Frank Beach Award Winner: Steroids as neuromodulators of brain circuits and behavior.Horm Behav. 2014 Aug;66(3):552-60. doi: 10.1016/j.yhbeh.2014.07.014. Epub 2014 Aug 7. Horm Behav. 2014. PMID: 25110187 Free PMC article. Review.
-
Removal of endogenous neuromodulators in a small motor network enhances responsiveness to neuromodulation.J Neurophysiol. 2017 Sep 1;118(3):1749-1761. doi: 10.1152/jn.00383.2017. Epub 2017 Jun 28. J Neurophysiol. 2017. PMID: 28659465 Free PMC article.
References
-
- Arimitsu T, Nuriya M, Ikeda K, Takahashi T, Yasui M. Activity-dependent regulation of HCN1 protein in cortical neurons. Biochem Biophys Res Commun. 2009;387:87–91. - PubMed
-
- Bal T, Nagy F, Moulins M. The pyloric central pattern generator in crustacea: a set of conditional neuronal oscillators. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1988;163:715–727.
-
- Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem. 2004;91:220–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
