Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats
- PMID: 22072691
- PMCID: PMC3236529
- DOI: 10.1523/JNEUROSCI.3280-11.2011
Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats
Abstract
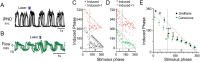
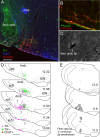
The retrotrapezoid nucleus contains Phox2b-expressing glutamatergic neurons (RTN-Phox2b neurons) that regulate breathing in a CO₂-dependent manner. Here we use channelrhodopsin-based optogenetics to explore how these neurons control breathing in conscious and anesthetized adult rats. Respiratory entrainment (pacing) of breathing frequency (fR) was produced over 57% (anesthetized) and 28% (conscious) of the natural frequency range by burst activation of RTN-Phox2b neurons (3-8 × 0.5-20 ms pulses at 20 Hz). In conscious rats, pacing under normocapnic conditions increased tidal volume (V(T)) and each inspiration was preceded by active expiration, denoting abdominal muscle contraction. During long-term pacing V(T) returned to prestimulation levels, suggesting that central chemoreceptors such as RTN-Phox2b neurons regulate V(T) partly independently of their effect on fR. Randomly applied light trains reset the respiratory rhythm and shortened the expiratory phase when the stimulus coincided with late-inspiration or early-expiration. Importantly, continuous (20 Hz) photostimulation of the RTN-Phox2b neurons and a saturating CO₂ concentration produced similar effects on breathing that were much larger than those elicited by phasic RTN stimulation. In sum, consistent with their anatomical projections, RTN-Phox2b neurons regulate lung ventilation by controlling breathing frequency, inspiration, and active expiration. Adult RTN-Phox2b neurons can entrain the respiratory rhythm if their discharge is artificially synchronized, but continuous activation of these neurons is much more effective at increasing lung ventilation. These results suggest that RTN-Phox2b neurons are no longer rhythmogenic in adulthood and that their average discharge rate may be far more important than their discharge pattern in driving lung ventilation.
Figures






References
-
- Ballanyi K, Ruangkittisakul A, Onimaru H. Opioids prolong and anoxia shortens delay between onset of preinspiratory (pFRG) and inspiratory (preBotC) network bursting in newborn rat brainstems. Pflugers Arch. 2009;458:571–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
