Potent autologous and heterologous neutralizing antibody responses occur in HIV-2 infection across a broad range of infection outcomes
- PMID: 22072758
- PMCID: PMC3255814
- DOI: 10.1128/JVI.06126-11
Potent autologous and heterologous neutralizing antibody responses occur in HIV-2 infection across a broad range of infection outcomes
Abstract
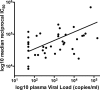
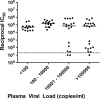
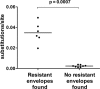
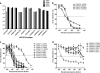
Few studies have explored the role of neutralizing antibody (NAb) responses in controlling HIV-2 viremia and disease progression. Using a TZM-bl neutralization assay, we assessed heterologous and autologous NAb responses from a community cohort of HIV-2-infected individuals with a broad range of disease outcomes in rural Guinea-Bissau. All subjects (n = 40) displayed exceptionally high heterologous NAb titers (50% inhibitory plasma dilution or 50% inhibitory concentration [IC(50)], 1:7,000 to 1:1,000,000) against 5 novel primary HIV-2 envelopes and HIV-2 7312A, whereas ROD A and 3 primary envelopes were relatively resistant to neutralization. Most individuals also showed high autologous NAb against contemporaneous envelopes (78% of plasma-envelope combinations in 69 envelopes from 21 subjects), with IC(50)s above 1:10,000. No association between heterologous or autologous NAb titer and greater control of HIV-2 was found. A subset of envelopes was found to be more resistant to neutralization (by plasma and HIV-2 monoclonal antibodies). These envelopes were isolated from individuals with greater intrapatient sequence diversity and were associated with changes in potential N-linked glycosylation sites but not CD4 independence or CXCR4 use. Plasma collected from up to 15 years previously was able to potently neutralize recent autologous envelopes, suggesting a lack of escape from NAb and the persistence of neutralization-sensitive variants over time, despite significant NAb pressure. We conclude that despite the presence of broad and potent NAb responses in HIV-2-infected individuals, these are not the primary forces behind the dichotomous outcomes observed but reveal a limited capacity for adaptive selection and escape from host immunity in HIV-2 infection.
Figures




References
-
- Aasa-Chapman MM, Aubin K, Williams I, McKnight A. 2006. Primary CCR5 only using HIV-1 isolates does not accurately represent the in vivo replicating quasi-species. Virology 351:489–496 - PubMed
-
- Abecasis AB, et al. 2010. Comparative performance of the REGA subtyping tool version 2 versus version 1. Infect. Genet. Evol. 10:380–385 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

