Development and optimization of oil-filled lipid nanoparticles containing docetaxel conjugates designed to control the drug release rate in vitro and in vivo
- PMID: 22072889
- PMCID: PMC3205148
- DOI: 10.2147/IJN.S24954
Development and optimization of oil-filled lipid nanoparticles containing docetaxel conjugates designed to control the drug release rate in vitro and in vivo
Abstract
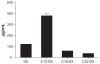
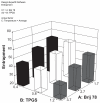
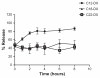
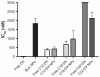
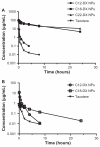
THREE DOCETAXEL (DX) LIPID CONJUGATES: 2'-lauroyl-docetaxel (C12-DX), 2'-stearoyl-docetaxel (C18-DX), and 2'-behenoyl-docetaxel (C22-DX) were synthesized to enhance drug loading, entrapment, and retention in liquid oil-filled lipid nanoparticles (NPs). The three conjugates showed ten-fold higher solubility in the liquid oil phase Miglyol 808 than DX. To further increase the drug entrapment efficiency in NPs, orthogonal design was performed. The optimized formulation was composed of Miglyol 808, Brij 78, and Vitamin E tocopheryl polyethylene glycol succinate (TPGS). The conjugates were successfully entrapped in the reduced-surfactant NPs with entrapment efficiencies of about 50%-60% as measured by gel permeation chromatography (GPC) at a final concentration of 0.5 mg/mL. All three conjugates showed 45% initial burst release in 100% mouse plasma. Whereas C12-DX showed another 40% release over the next 8 hours, C18-DX and C22-DX in NPs showed no additional release after the initial burst of drug. All conjugates showed significantly lower cytotoxicity than DX in human DU-145 prostate cancer cells. The half maximal inhibitory concentration values (IC(50)) of free conjugates and conjugate NPs were comparable except for C22-DX, which was nontoxic in the tested concentration range and showed only vehicle toxicity when entrapped in NPs. In vivo, the total area under the curve (AUC(0-∞)) values of all DX conjugate NPs were significantly greater than that of Taxotere, demonstrating prolonged retention of drug in the blood. The AUC(0-∞) value of DX in Taxotere was 8.3-fold, 358.0-fold, and 454.5-fold lower than that of NP-formulated C12-DX, C18-DX, and C22-DX, respectively. The results of these studies strongly support the idea that the physical/chemical properties of DX conjugates may be fine-tuned to influence the affinity and retention of DX in oil-filled lipid NPs, which leads to very different pharmacokinetic profiles and blood exposure of an otherwise potent chemo-therapeutic agent. These studies and methodologies may allow for improved and more potent nanoparticle-based formulations.
Keywords: docetaxel; ester prodrug; nanoparticles; sustained release.
Figures
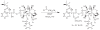

References
-
- Goldspiel BR. Clinical overview of the taxanes. Pharmacotherapy. 1997;17(5 Pt 2):110S–125S. - PubMed
-
- van Oosterom AT, Schrijvers D, Schrijvers D. Docetaxel (Taxotere), a review of preclinical and clinical experience. Part II: Clinical experience. Anticancer Drugs. 1995;6(3):356–368. - PubMed
-
- Cortes JE, Pazdur R. Docetaxel. J Clin Oncol. 1995;13(10):2643–2655. - PubMed
-
- Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991;83(4):288–291. - PubMed
-
- Extra JM, Rousseau F, Bruno R, Clavel M, Le Bail N, Marty M. Phase I and pharmacokinetic study of Taxotere (RP56976;NSC 628503) given as a short intravenous infusion. Cancer Res. 1993;53(5):1037–1042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

