Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes
- PMID: 22072969
- PMCID: PMC3207916
- DOI: 10.1371/journal.ppat.1002351
Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes
Abstract
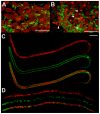
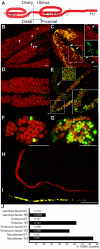
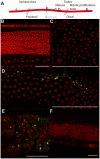
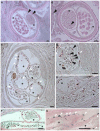
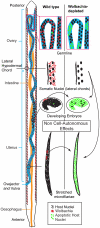
Filarial nematodes maintain a mutualistic relationship with the endosymbiont Wolbachia. Depletion of Wolbachia produces profound defects in nematode development, fertility and viability and thus has great promise as a novel approach for treating filarial diseases. However, little is known concerning the basis for this mutualistic relationship. Here we demonstrate using whole mount confocal microscopy that an immediate response to Wolbachia depletion is extensive apoptosis in the adult germline, and in the somatic cells of the embryos, microfilariae and fourth-stage larvae (L4). Surprisingly, apoptosis occurs in the majority of embryonic cells that had not been infected prior to antibiotic treatment. In addition, no apoptosis occurs in the hypodermal chords, which are populated with large numbers of Wolbachia, although disruption of the hypodermal cytoskeleton occurs following their depletion. Thus, the induction of apoptosis upon Wolbachia depletion is non-cell autonomous and suggests the involvement of factors originating from Wolbachia in the hypodermal chords. The pattern of apoptosis correlates closely with the nematode tissues and processes initially perturbed following depletion of Wolbachia, embryogenesis and long-term sterilization, which are sustained for several months until the premature death of the adult worms. Our observations provide a cellular mechanism to account for the sustained reductions in microfilarial loads and interruption of transmission that occurs prior to macrofilaricidal activity following antibiotic therapy of filarial nematodes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



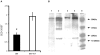
References
-
- Kozek WJ. Transovarially-transmitted intracellular microorganisms in adult and larval stages of Brugia malayi. J Parasitol. 1977;63:992–1000. - PubMed
-
- Kozek WJ, Marroquin HF. Intracytoplasmic bacteria in Onchocerca volvulus. Am J Trop Med Hyg. 1977;26:663–678. - PubMed
-
- Taylor MJ, Hoerauf A. Wolbachia bacteria of filarial nematodes. Parasitol Today. 1999;15:437–442. - PubMed
-
- Taylor MJ, Bilo K, Cross HF, Archer JP, Underwood AP. 16S rDNA phylogeny and ultrastructural characterization of Wolbachia intracellular bacteria of the filarial nematodes Brugia malayi, B. pahangi, and Wuchereria bancrofti. Exp Parasitol. 1999;91:356–361. - PubMed
-
- Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175–1185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

