Biomaterials in cochlear implants
- PMID: 22073103
- PMCID: PMC3199815
- DOI: 10.3205/cto000062
Biomaterials in cochlear implants
Abstract
The cochlear implant (CI) represents, for almost 25 years now, the gold standard in the treatment of children born deaf and for postlingually deafened adults. These devices thus constitute the greatest success story in the field of 'neurobionic' prostheses. Their (now routine) fitting in adults, and especially in young children and even babies, places exacting demands on these implants, particularly with regard to the biocompatibility of a CI's surface components. Furthermore, certain parts of the implant face considerable mechanical challenges, such as the need for the electrode array to be flexible and resistant to breakage, and for the implant casing to be able to withstand external forces.As these implants are in the immediate vicinity of the middle-ear mucosa and of the junction to the perilymph of the cochlea, the risk exists - at least in principle - that bacteria may spread along the electrode array into the cochlea. The wide-ranging requirements made of the CI in terms of biocompatibility and the electrode mechanism mean that there is still further scope - despite the fact that CIs are already technically highly sophisticated - for ongoing improvements to the properties of these implants and their constituent materials, thus enhancing the effectiveness of these devices.This paper will therefore discuss fundamental material aspects of CIs as well as the potential for their future development.
Keywords: biocompatibility; biomaterials; coating; cochlear implant; cochleostomy; drug delivery; electrode; inner ear; nanoparticles; surface functionalization.
Figures

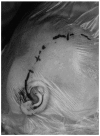
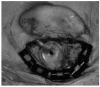

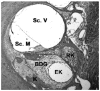
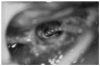


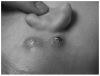
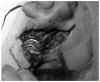
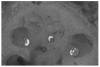
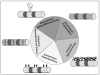






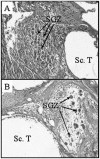

References
-
- Burgio P. Safety considerations of cochlear implantation. Otolaryngol Clin North Am. 1986;19(2):237–247. - PubMed
-
- Lehnhardt E. Biokompatibilität der Cochlear-implants. Eur Arch Otorhinolaryngol Suppl. 1992;1:223–233. - PubMed
-
- BQS Qualitätsreport. Düsseldorf: BQS Institut für Qualität und Patientensicherheit; 2007. p. 36. Available from: http://www.bqs-qualitaetsreport.de/
-
- Dunn CC, Tyler RS, Oakley S, Gantz BJ, Noble W. Comparison of speech recognition and localization performance in bilateral and unilateral cochlear implant users matched on duration of deafness and age at implantation. Ear Hear. 2008;29(3):352–359. doi: 10.1097/AUD.0b013e318167b870. Available from: http://dx.doi.org/10.1097/AUD.0b013e318167b870. - DOI - PMC - PubMed
-
- O'Donoghue GM, Nikolopoulos TP. Minimal access surgery for pediatric cochlear implantation. Otol Neurotol. 2002;23(6):891–894. doi: 10.1097/00129492-200211000-00014. Available from: http://dx.doi.org/10.1097/00129492-200211000-00014. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

