The prevalence of injection-site reactions with disease-modifying therapies and their effect on adherence in patients with multiple sclerosis: an observational study
- PMID: 22074056
- PMCID: PMC3227610
- DOI: 10.1186/1471-2377-11-144
The prevalence of injection-site reactions with disease-modifying therapies and their effect on adherence in patients with multiple sclerosis: an observational study
Abstract
Background: Interferon beta (IFNβ) and glatiramer acetate (GA) are administered by subcutaneous (SC) or intramuscular (IM) injection. Patients with multiple sclerosis (MS) often report injection-site reactions (ISRs) as a reason for noncompliance or switching therapies. The aim of this study was to compare the proportion of patients on different formulations of IFNβ or GA who experienced ISRs and who switched or discontinued therapy because of ISRs.
Methods: The Swiss MS Skin Project was an observational multicenter study. Patients with MS or clinically isolated syndrome who were on the same therapy for at least 2 years were enrolled. A skin examination was conducted at the first study visit and 1 year later.
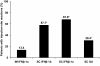
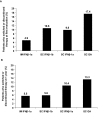
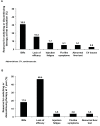
Results: The 412 patients enrolled were on 1 of 4 disease-modifying therapies for at least 2 years: IM IFNβ-1a (n = 82), SC IFNβ-1b (n = 123), SC IFNβ-1a (n = 184), or SC GA (n = 23). At first evaluation, ISRs were reported by fewer patients on IM IFNβ-1a (13.4%) than on SC IFNβ-1b (57.7%; P < 0.0001), SC IFNβ-1a (67.9%; P < 0.0001), or SC GA (30.4%; P = not significant [NS]). No patient on IM IFNβ-1a missed a dose in the previous 4 weeks because of ISRs, compared with 5.7% of patients on SC IFNβ-1b (P = 0.044), 7.1% of patients on SC IFNβ-1a (P = 0.011), and 4.3% of patients on SC GA (P = NS). Primary reasons for discontinuing or switching therapy were ISRs or lack of efficacy. Similar patterns were observed at 1 year.
Conclusions: Patients on IM IFNβ-1a had fewer ISRs and were less likely to switch therapies than patients on other therapies. This study may have implications in selecting initial therapy or, for patients considering switching or discontinuing therapy because of ISRs, selecting an alternative option.
Figures
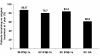
References
-
- Panitch H, Goodin DS, Francis G, Chang P, Coyle PK, O'Connor P, Monaghan E, Li D, Weinshenker B. EVIDENCE (EVidence of Interferon Dose-response: European North American Comparative Efficacy) Study Group; University of British Columbia MS/MRI Research Group. Randomized, comparative study of interferon β-1a treatment regimens in MS: the EVIDENCE trial. Neurology. 2002;59(10):1496–1506. - PubMed
-
- Durelli L, Verdun E, Barbero P, Bergui M, Versino E, Ghezzi A, Montanari E, Zaffaroni M. Independent Comparison of Interferon (INCOMIN) Trial Study Group. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN) Lancet. 2002;359(9316):1453–1460. doi: 10.1016/S0140-6736(02)08430-1. - DOI - PubMed
-
- Milanese C, La Mantia L, Palumbo R, Martinelli V, Murialdo A, Zaffaroni M, Caputo D, Capra R, Bergamaschi R. the North Italy Multiple Sclerosis Group. A post-marketing study on interferon β 1b and 1a treatment in relapse-remitting multiple sclerosis: different response in drop-outs and treated patients. J Neurol Neurosurg Psychiatry. 2003;74:1689–1692. doi: 10.1136/jnnp.74.12.1689. - DOI - PMC - PubMed
-
- O'Connor P, Filippi M, Arnason B, Comi G, Cook S, Goodin D, Hartung HP, Jeffery D, Kappos L, Boateng F, Filippov V, Groth M, Knappertz V, Christian Kraus C, Sandbrink R, Pohl C, Bogumil T. for the BEYOND Study Group. 250 μg or 500 μg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomized, multicentre study. Lancet Neurol. 2009;8(10):889–897. doi: 10.1016/S1474-4422(09)70226-1. - DOI - PubMed
-
- Tremlett HL, Oger J. Interrupted therapy: stopping and switching of the beta-interferons prescribed for MS. Neurology. 2003;61(4):551–554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

