Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator
- PMID: 22074219
- PMCID: PMC3305913
- DOI: 10.1186/1475-2875-10-339
Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator
Abstract
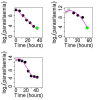
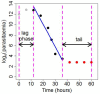
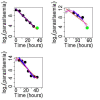
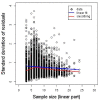
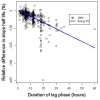
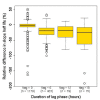
Background: A significant reduction in parasite clearance rates following artesunate treatment of falciparum malaria, and increased failure rates following artemisinin combination treatments (ACT), signaled emergent artemisinin resistance in Western Cambodia. Accurate measurement of parasite clearance is therefore essential to assess the spread of artemisinin resistance in Plasmodium falciparum. The slope of the log-parasitaemia versus time relationship is considered to be the most robust measure of anti-malarial effect. However, an initial lag phase of numerical instability often precedes a steady exponential decline in the parasite count after the start of anti-malarial treatment. This lag complicates the clearance estimation, introduces observer subjectivity, and may influence the accuracy and consistency of reported results.
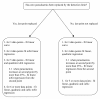
Methods: To address this problem, a new approach to modelling clearance of malaria parasites from parasitaemia-time profiles has been explored and validated. The methodology detects when a lag phase is present, selects the most appropriate model (linear, quadratic or cubic) to fit log-transformed parasite data, and calculates estimates of parasite clearance adjusted for this lag phase. Departing from previous approaches, parasite counts below the level of detection are accounted for and not excluded from the calculation.
Results: Data from large clinical studies with frequent parasite counts were examined. The effect of a lag phase on parasite clearance rate estimates is discussed, using individual patient data examples. As part of the World Wide Antimalarial Resistance Network's (WWARN) efforts to make innovative approaches available to the malaria community, an automated informatics tool: the parasite clearance estimator has been developed.
Conclusions: The parasite clearance estimator provides a consistent, reliable and accurate method to estimate the lag phase and malaria parasite clearance rate. It could be used to detect early signs of emerging resistance to artemisinin derivatives and other compounds which affect ring-stage clearance.
Figures



References
-
- World Health Organization. Guidelines for the treatment of malaria, second edition. World Health Organization, Geneva; 2010.
-
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

