Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis
- PMID: 22074258
- PMCID: PMC3229459
- DOI: 10.1186/1471-244X-11-176
Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis
Abstract
Background: Psychostimulants and non stimulants are effective in the treatment of ADHD. Efficacy of both methylphenidate and atomoxetine has been established in placebo controlled trials. Direct comparison of efficacy is now possible due to availability of results from several head-to-head trials of these two medications.
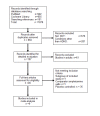
Methods: All published, randomized, open label or double blind trials, comparing efficacy of methylphenidate with atomoxetine, in treatment of ADHD in children, diagnosed using DSM-IV™ criteria were included. The outcome studied was ADHDRS-IVParent:Inv score. The standardized mean difference (SMD) was used as a measure of effect size.
Results: Nine randomized trials comparing methylphenidate and atomoxetine, with a total of 2762 participants were included. Meta-analysis did not find a significant difference in efficacy between methylphenidate and atomoxetine (SMD=0.09, 95% CI -0.08-0.26) (Z=1.06, p=0.29). Synthesis of data from eight trials found no significant difference in response rates (RR=0.93 95% CI 0.76-1.14, p=0.49). Sub group analysis showed a significant standardized mean difference favouring OROS methylphenidate (SMD=0.32, 95% CI 0.12-0.53 (Z=3.05, p<0.002). Immediate release methylphenidate was not superior to atomoxetine (SMD=-0.04, 95% CI -0.19-0.12) (Z=0.46, p=0.64). Excluding open label trials did not significantly alter the effect size (SMD=0.08, 95% CI -0.04-0.21) (Z=1.27, p=0.20). All-cause discontinuation was used as a measure of acceptability. There was no significant difference in all cause discontinuation between atomoxetine and methylphenidate (RR 1.22, 95% CI 0.87-1.71). There was significant heterogeneity among the studies (p=0.002, I2=67%). Subgroup analysis demonstrated the heterogeneity to be due to the open label trials (p=0.001, I2=81%).
Conclusions: In general atomoxetine and methylphenidate have comparable efficacy and equal acceptability in treatment of ADHD in children and adolescents. However OROS methylphenidate is more effective than atomoxetine and may be considered as first line treatment in treatment of ADHD in children and adolescents.
Figures
Comment in
-
Review: methylphenidate and atomoxetine have similar efficacy and acceptability in children and adolescents with ADHD.Evid Based Ment Health. 2012 Aug;15(3):74. doi: 10.1136/ebmental-2012-100717. Epub 2012 May 11. Evid Based Ment Health. 2012. PMID: 22581018 No abstract available.
References
-
- American Association of Pediatrics. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. American Academy of Pediatrics. Pediatrics. 2000;105(5):1158–1170. - PubMed
-
- Centers for Disease Control and Prevention. Mental health in the United States. Prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder--United States, 2003. MMWR Morb Mortal Wkly Rep. 2005;54(34):842–847. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical