Microglia use multiple mechanisms to mediate interactions with vitronectin; non-essential roles for the highly-expressed αvβ3 and αvβ5 integrins
- PMID: 22074485
- PMCID: PMC3239846
- DOI: 10.1186/1742-2094-8-157
Microglia use multiple mechanisms to mediate interactions with vitronectin; non-essential roles for the highly-expressed αvβ3 and αvβ5 integrins
Abstract
Background: As the primary resident immune cells, microglia play a central role in regulating inflammatory processes in the CNS. The extracellular matrix (ECM) protein vitronectin promotes microglial activation, switching microglia into an activated phenotype. We have shown previously that microglia express two vitronectin receptors, αvβ3 and αvβ5 integrins. As these integrins have well-defined roles in activation and phagocytic processes in other cell types, the purpose of the current study was to investigate the contribution of these two integrins in microglial activation.
Methods: Microglial cells were prepared from wild-type, β3 integrin knockout (KO), β5 integrin KO or β3/β5 integrin DKO mice, and their interactions and activation responses to vitronectin examined in a battery of assays, including adhesion, expression of activation markers, MMP-9 expression, and phagocytosis. Expression of other αv integrins was examined by flow cytometry and immunoprecipitation.
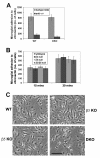
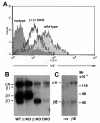
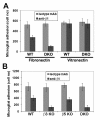
Results: Surprisingly, when cultured on vitronectin, microglia from the different knockout strains showed no obvious defects in adhesion, activation marker expression, MMP-9 induction, or phagocytosis of vitronectin-coated beads. To investigate the reason for this lack of effect, we examined the expression of other αv integrins. Flow cytometry showed that β3/β5 integrin DKO microglia expressed residual αv integrin at the cell surface, and immunoprecipitation confirmed this finding by revealing the presence of low levels of the αvβ1 and αvβ8 integrins. β1 integrin blockade had no impact on adhesion of β3/β5 integrin DKO microglia to vitronectin, suggesting that in addition to αvβ1, αvβ3, and αvβ5, αvβ8 also serves as a functional vitronectin receptor on microglia.
Conclusions: Taken together, this demonstrates that the αvβ3 and αvβ5 integrins are not essential for mediating microglial activation responses to vitronectin, but that microglia use multiple redundant receptors to mediate interactions with this ECM protein.
Figures


References
-
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev. 1999;30:77–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

