Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomised trial
- PMID: 22074711
- PMCID: PMC3213241
- DOI: 10.1136/bmj.d6792
Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomised trial
Abstract
Objective: To evaluate the use of routine laboratory monitoring in terms of clinical outcomes among patients receiving antiretroviral therapy (ART) in Uganda.
Design: Randomised clinical trial
Setting: A home based ART programme in rural Uganda.
Participants: All participants were people with HIV who were members of the AIDS Support Organisation. Participants had CD4 cell counts <250 cells × 10(6)/L or World Health Organization stage 3 or 4 disease.
Interventions: Participants were randomised to one of three different monitoring arms: a viral load arm (clinical monitoring, quarterly CD4 counts, and viral load measurements), CD4 arm (clinical monitoring and CD4 counts), or clinical arm (clinical monitoring alone).
Main outcome measures: Serious morbidity (newly diagnosed AIDS defining illness) and mortality.
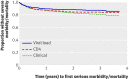
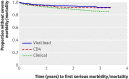
Results: 1094 participants started ART; median CD4 count at baseline was 129 cells × 10(6)/L. Median follow-up was three years. In total, 126 participants died (12%), 148 (14%) experienced new AIDS defining illnesses, and 61(6%) experienced virological failure, defined as two consecutive viral loads >500 copies/mL occurring more than three months after the start of ART. After adjustment for age, sex, baseline CD4 count, viral load, and body mass index, the rate of new AIDS defining events or death was higher in the clinical arm than the viral load arm (adjusted hazard ratio 1.83, P = 0.002) or the CD4 arm (1.49, P = 0.032). There was no significant difference between the CD4 arm and the viral load arm (1.23, P = 0.31).
Conclusion: In patients receiving ART for HIV infection in Uganda, routine laboratory monitoring is associated with improved health and survival compared with clinical monitoring alone. Trial registration Clinical Trials NCT00119093.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Ethical approval: The studies were approved by the Institutional Review Boards of the Centers for Disease Control and Prevention and the Uganda Virus Research Institute and informed consent was given by all patients.
Figures

Comment in
-
Antiretroviral therapy programmes in resource limited settings.BMJ. 2011 Nov 9;343:d6853. doi: 10.1136/bmj.d6853. BMJ. 2011. PMID: 22074712 No abstract available.
References
-
- UNAIDS. Report on the global AIDS epidemic 2010. UNAIDS, 2010.
-
- Samb B, Celletti F, Holloway J, Van Damme W, De Cock KM, Dybul M. Rapid expansion of the health workforce in response to the HIV epidemic. N Engl J Med 2007;357:2510-4. - PubMed
-
- Kumarasamy N. Generic antiretroviral drugs—will they be the answer to HIV in the developing world? Lancet 2004;364:3-4. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
