CD4 cell count and viral load monitoring in patients undergoing antiretroviral therapy in Uganda: cost effectiveness study
- PMID: 22074713
- PMCID: PMC3213243
- DOI: 10.1136/bmj.d6884
CD4 cell count and viral load monitoring in patients undergoing antiretroviral therapy in Uganda: cost effectiveness study
Abstract
Objective: To examine the cost and cost effectiveness of quarterly CD4 cell count and viral load monitoring among patients taking antiretroviral therapy (ART).
Design: Cost effectiveness study.
Setting: A randomised trial in a home based ART programme in Tororo, Uganda.
Participants: People with HIV who were members of the AIDS Support Organisation and had CD4 cell counts <250 × 10(6) cells/L or World Health Organization stage 3 or 4 disease.
Main outcome measures: Outcomes calculated for the study period and projected 15 years into the future included costs, disability adjusted life years (DALYs), and incremental cost effectiveness ratios (ICER; $ per DALY averted). Cost inputs were based on the trial and other sources. Clinical inputs derived from the trial; in the base case, we assumed that point estimates reflected true differences even if non-significant. We conducted univariate and multivariate sensitivity analyses.
Interventions: Three monitoring strategies: clinical monitoring with quarterly CD4 cell counts and viral load measurement (clinical/CD4/viral load); clinical monitoring and quarterly CD4 counts (clinical/CD4); and clinical monitoring alone.
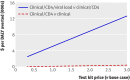
Results: With the intention to treat (ITT) results per 100 individuals starting ART, we found that clinical/CD4 monitoring compared with clinical monitoring alone increases costs by $20,458 (£12,780, €14,707) and averts 117.3 DALYs (ICER = $174 per DALY). Clinical/CD4/viral load monitoring compared with clinical/CD4 monitoring adds $142,458, and averts 27.5 DALYs ($5181 per DALY). The superior ICER for clinical/CD4 monitoring is robust to uncertainties in input values, and that strategy is dominant (less expensive and more effective) compared with clinical/CD4/viral load monitoring in one quarter of simulations. If clinical inputs are based on the as treated analysis starting at 90 days (after laboratory monitoring was initiated), then clinical/CD4/viral load monitoring is dominated by other strategies.
Conclusions: Based on this trial, compared with clinical monitoring alone, monitoring of routine CD4 cell count is considerably more cost effective than additionally including routine viral load testing in the monitoring strategy and is more cost effective than ART.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Antiretroviral therapy programmes in resource limited settings.BMJ. 2011 Nov 9;343:d6853. doi: 10.1136/bmj.d6853. BMJ. 2011. PMID: 22074712 No abstract available.
References
-
- UNAIDS. 2010 report on the global HIV/AIDS epidemic. UNAIDS, 2010. www.cia.gov/library/publications/the-world-factbook/geos/ug.html.
-
- Mermin J, Were W, Ekwaru JP, Moore D, Downing R, Behumbiize P, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet 2008;371:752-9. - PubMed
-
- Tweya H, Gareta D, Chagwera F, Ben-Smith A, Mwenyemasi J, Chiputula F, et al. Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the ‘Back-to-Care’ project in Lilongwe, Malawi. Trop Med Int Health 2010;15(suppl 1):82-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials