Three-dimensional imaging and photostimulation by remote-focusing and holographic light patterning
- PMID: 22074779
- PMCID: PMC3241782
- DOI: 10.1073/pnas.1109111108
Three-dimensional imaging and photostimulation by remote-focusing and holographic light patterning
Abstract
Access to three-dimensional structures in the brain is fundamental to probe signal processing at multiple levels, from integration of synaptic inputs to network activity mapping. Here, we present an optical method for independent three-dimensional photoactivation and imaging by combination of digital holography with remote-focusing. We experimentally demonstrate compensation of spherical aberration for out-of-focus imaging in a range of at least 300 μm, as well as scanless imaging along oblique planes. We apply this method to perform functional imaging along tilted dendrites of hippocampal pyramidal neurons in brain slices, after photostimulation by multiple spots glutamate uncaging. By bringing extended portions of tilted dendrites simultaneously in-focus, we monitor the spatial extent of dendritic calcium signals, showing a shift from a widespread to a spatially confined response upon blockage of voltage-gated Na(+) channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


 ), first-order (
), first-order ( ), and second-order (
), and second-order ( ) spherical aberrations, measured with the setup of A, experimental data (blue triangles and black squares, for O1 and O2, respectively) were in agreement with theoretical predictions (blue and black lines, for O1 and O2, respectively) obtained in the linear approximation for N.A.O1 = 0.76 and N.A.O2 = 0.72. (C) Remote-focusing setup modified for aberration measurements. L1-L4, achromatic lenses [focal lengths (millimeter), fL1 = 200; fL2–L3 = 150; fL4 = 100]. (D, Top and Middle). Zernike coefficients for defocus (
) spherical aberrations, measured with the setup of A, experimental data (blue triangles and black squares, for O1 and O2, respectively) were in agreement with theoretical predictions (blue and black lines, for O1 and O2, respectively) obtained in the linear approximation for N.A.O1 = 0.76 and N.A.O2 = 0.72. (C) Remote-focusing setup modified for aberration measurements. L1-L4, achromatic lenses [focal lengths (millimeter), fL1 = 200; fL2–L3 = 150; fL4 = 100]. (D, Top and Middle). Zernike coefficients for defocus ( ) and first-order spherical aberration (
) and first-order spherical aberration ( ) introduced by O1 (blue triangles), O2 (black squares) were measured in the setup of C by moving MO or MR, respectively. Then, defocus was canceled (Top, red circles) by moving MO and MR simultaneously. First-order spherical aberration was almost perfectly compensated (Middle, red circles) and could be well approximated by summing (red line) the linear fits of first-order spherical aberrations introduced by O1 and O2 (blue and black lines, respectively), in agreement with theory. (Bottom) Measured Strehl ratio for uncompensated (blue triangles) and compensated (red circles) configurations. The green star corresponds to the Strehl ratio measured in setup A with objective O1 (without the remote-focusing unit).
) introduced by O1 (blue triangles), O2 (black squares) were measured in the setup of C by moving MO or MR, respectively. Then, defocus was canceled (Top, red circles) by moving MO and MR simultaneously. First-order spherical aberration was almost perfectly compensated (Middle, red circles) and could be well approximated by summing (red line) the linear fits of first-order spherical aberrations introduced by O1 and O2 (blue and black lines, respectively), in agreement with theory. (Bottom) Measured Strehl ratio for uncompensated (blue triangles) and compensated (red circles) configurations. The green star corresponds to the Strehl ratio measured in setup A with objective O1 (without the remote-focusing unit).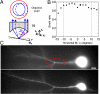

References
-
- London M, Hausser M. Dendritic computation. Annu Rev Neurosci. 2005;28:503–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

