Photoreceptor signaling: supporting vision across a wide range of light intensities
- PMID: 22074925
- PMCID: PMC3265842
- DOI: 10.1074/jbc.R111.305243
Photoreceptor signaling: supporting vision across a wide range of light intensities
Abstract
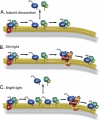
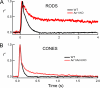
For decades, photoreceptors have been an outstanding model system for elucidating basic principles in sensory transduction and biochemistry and for understanding many facets of neuronal cell biology. In recent years, new knowledge of the kinetics of signaling and the large-scale movements of proteins underlying signaling has led to a deeper appreciation of the photoreceptor's unique challenge in mediating the first steps in vision over a wide range of light intensities.
Figures

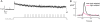
References
-
- Burns M. E., Baylor D. A. (2001) Annu. Rev. Neurosci. 24, 779–805 - PubMed
-
- Fain G. L., Matthews H. R., Cornwall M. C., Koutalos Y. (2001) Physiol. Rev. 81, 117–151 - PubMed
-
- Arshavsky V. Y., Lamb T. D., Pugh E. N., Jr. (2002) Annu. Rev. Physiol. 64, 153–187 - PubMed
-
- Maeda T., Imanishi Y., Palczewski K. (2003) Prog. Retin. Eye Res. 22, 417–434 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

