Focal adhesion kinase inhibitors are potent anti-angiogenic agents
- PMID: 22075057
- PMCID: PMC5528320
- DOI: 10.1016/j.molonc.2011.10.004
Focal adhesion kinase inhibitors are potent anti-angiogenic agents
Abstract
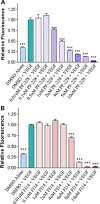
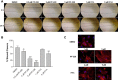
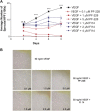
Focal adhesion kinase (FAK), a cytoplasmic tyrosine kinase and scaffold protein localized to focal adhesions, is uniquely positioned at the convergence point of integrin and receptor tyrosine kinase signal transduction pathways. FAK is overexpressed in many tumor cells, hence various inhibitors targeting its activity have been tested for anti-tumor activity. However, the direct effects of these pharmacologic agents on the endothelial cells of the vasculature have not been examined. Using primary human umbilical vein endothelial cells (HUVEC), we characterized the effects of two FAK inhibitors, PF-573,228 and FAK Inhibitor 14 on essential processes for angiogenesis, such as migration, proliferation, viability and endothelial cell tube formation. We observed that treatment with either FAK Inhibitor 14 or PF-573,228 resulted in reduced HUVEC viability, migration and tube formation in response to vascular endothelial growth factor (VEGF). Furthermore, we found that PF-573,228 had the added ability to induce apoptosis of endothelial cells within 36 h post-drug administration even in the continued presence of VEGF stimulation. FAK inhibitors also resulted in modification of the actin cytoskeleton within HUVEC, with observed increased stress fiber formation in the presence of drug. Given that endothelial cells were sensitive to FAK inhibitors at concentrations well below those reported to inhibit tumor cell migration, we confirmed their ability to inhibit endothelial-derived FAK autophosphorylation and FAK-mediated phosphorylation of recombinant paxillin at these doses. Taken together, our data indicate that small molecule inhibitors of FAK are potent anti-angiogenic agents and suggest their utility in combinatorial therapeutic approaches targeting tumor angiogenesis.
Copyright © 2011 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Abedi, H. , Zachary, I. , 1997. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. The Journal of Biological Chemistry. 272, 15442–15451. - PubMed
-
- Abu-Ghazaleh, R. , Kabir, J. , Jia, H. , Lobo, M. , Zachary, I. , 2001. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. The Biochemical Journal. 360, 255–264. - PMC - PubMed
-
- Bagi, C.M. , Christensen, J. , Cohen, D.P. , Roberts, W.G. , Wilkie, D. , Swanson, T. , Tuthill, T. , Andresen, C.J. , 2009. Sunitinib and PF-562,271 (FAK/Pyk2 inhibitor) effectively block growth and recovery of human hepatocellular carcinoma in a rat xenograft model. Cancer Biology & Therapy. 8, 856–865. - PubMed
-
- Bagi, C.M. , Roberts, G.W. , Andresen, C.J. , 2008. Dual focal adhesion kinase/Pyk2 inhibitor has positive effects on bone tumors: implications for bone metastases. Cancer. 112, 2313–2321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

