A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes
- PMID: 22075145
- PMCID: PMC3235737
- DOI: 10.1016/j.devcel.2011.09.005
A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes
Abstract
Polyphosphoinositides are lipid signaling molecules generated from phosphatidylinositol (PtdIns) with critical roles in vesicular trafficking and signaling. It is poorly understood where PtdIns is located within cells and how it moves around between membranes. Here we identify a hitherto-unrecognized highly mobile membrane compartment as the site of PtdIns synthesis and a likely source of PtdIns of all membranes. We show that the PtdIns-synthesizing enzyme PIS associates with a rapidly moving compartment of ER origin that makes ample contacts with other membranes. In contrast, CDP-diacylglycerol synthases that provide PIS with its substrate reside in the tubular ER. Expression of a PtdIns-specific bacterial PLC generates diacylglycerol also in rapidly moving cytoplasmic objects. We propose a model in which PtdIns is synthesized in a highly mobile lipid distribution platform and is delivered to other membranes during multiple contacts by yet-to-be-defined lipid transfer mechanisms.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
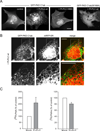
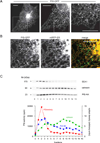

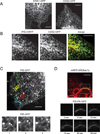
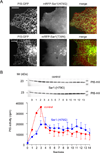
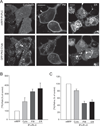
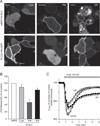
Comment in
-
Phosphatidylinositol synthase and diacylglycerol platforms bust a move.Dev Cell. 2011 Nov 15;21(5):810-2. doi: 10.1016/j.devcel.2011.10.016. Dev Cell. 2011. PMID: 22075144 Free PMC article.
Similar articles
-
Independent phosphatidylinositol synthesis in pituitary plasma membrane and endoplasmic reticulum.Nature. 1987 Feb 19-25;325(6106):726-8. doi: 10.1038/325726a0. Nature. 1987. PMID: 3029593
-
Phosphatidylinositol and phosphatidic acid transport between the ER and plasma membrane during PLC activation requires the Nir2 protein.Biochem Soc Trans. 2016 Feb;44(1):197-201. doi: 10.1042/BST20150187. Biochem Soc Trans. 2016. PMID: 26862206 Free PMC article. Review.
-
Assessment of mitochondria as a compartment for phosphatidylinositol synthesis in Solanum tuberosum.Plant Physiol Biochem. 2010 Dec;48(12):952-60. doi: 10.1016/j.plaphy.2010.09.004. Epub 2010 Sep 18. Plant Physiol Biochem. 2010. PMID: 20947365
-
Neuronal ER-plasma membrane junctions organized by Kv2-VAP pairing recruit Nir proteins and affect phosphoinositide homeostasis.J Biol Chem. 2019 Nov 22;294(47):17735-17757. doi: 10.1074/jbc.RA119.007635. Epub 2019 Oct 8. J Biol Chem. 2019. PMID: 31594866 Free PMC article.
-
Features of the Phosphatidylinositol Cycle and its Role in Signal Transduction.J Membr Biol. 2017 Aug;250(4):353-366. doi: 10.1007/s00232-016-9909-y. Epub 2016 Jun 8. J Membr Biol. 2017. PMID: 27278236 Review.
Cited by
-
Methods to study phosphoinositide regulation of ion channels.Methods Enzymol. 2021;652:49-79. doi: 10.1016/bs.mie.2021.01.025. Epub 2021 Mar 4. Methods Enzymol. 2021. PMID: 34059290 Free PMC article.
-
Nonvesicular lipid transfer from the endoplasmic reticulum.Cold Spring Harb Perspect Biol. 2012 Oct 1;4(10):a013300. doi: 10.1101/cshperspect.a013300. Cold Spring Harb Perspect Biol. 2012. PMID: 23028121 Free PMC article. Review.
-
Rab10 joins the ER social network.Nat Cell Biol. 2013 Feb;15(2):135-6. doi: 10.1038/ncb2682. Nat Cell Biol. 2013. PMID: 23377026
-
Chemical Approaches for Measuring and Manipulating Lipids at the Organelle Level.Cold Spring Harb Perspect Biol. 2023 Dec 1;15(12):a041407. doi: 10.1101/cshperspect.a041407. Cold Spring Harb Perspect Biol. 2023. PMID: 37604586 Free PMC article. Review.
-
Looking Beyond Structure: Membrane Phospholipids of Skeletal Muscle Mitochondria.Trends Endocrinol Metab. 2016 Aug;27(8):553-562. doi: 10.1016/j.tem.2016.05.007. Epub 2016 Jun 28. Trends Endocrinol Metab. 2016. PMID: 27370525 Free PMC article. Review.
References
-
- Acharya JK, Labarca P, Delgado R, Jalink K, Zuker CS. Synaptic defects and compensatory regulation of inositol metabolism in inositol polyphosphate 1-phosphatase mutants. Neuron. 1998;20:1219–1229. - PubMed
-
- Agranoff BW, Bradley RM, Brady RO. The enzymatic synthesis of inositol phosphatide. J.Biol.Chem. 1958;233:1077–1083. - PubMed
-
- Balla T, Baukal AJ, Guillemette G, Catt KJ. Multiple pathways of inositol polyphosphate metabolism in angiotensin-stimulated adrenal glomerulosa cells. J.Biol.Chem. 1988;263:4083–4091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

