Copper: an essential metal in biology
- PMID: 22075424
- PMCID: PMC3718004
- DOI: 10.1016/j.cub.2011.09.040
Copper: an essential metal in biology
Figures
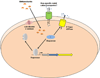
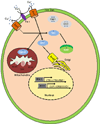
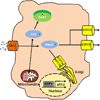
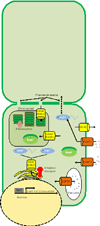
References
-
- Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–185. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

