A-kinase anchoring proteins that regulate cardiac remodeling
- PMID: 22075671
- PMCID: PMC3475412
- DOI: 10.1097/FJC.0b013e31821c0220
A-kinase anchoring proteins that regulate cardiac remodeling
Abstract
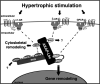
In response to injury or stress, the adult heart undergoes maladaptive changes, collectively defined as pathological cardiac remodeling. Here, we focus on the role of A-kinase anchoring proteins (AKAPs) in 3 main areas associated with cardiac remodeling and the progression of heart failure: excitation-contraction coupling, sarcomeric regulation, and induction of pathological hypertrophy. AKAPs are a diverse family of scaffold proteins that form multiprotein complexes, integrating cAMP signaling with protein kinases, phosphatases, and other effector proteins. Many AKAPs have been characterized in the heart, where they play a critical role in modulating cardiac function.
Figures


References
-
- Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. - PubMed
-
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. NatRev MolCellBiol. 2006;7:589–600. - PubMed
-
- Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. - PubMed
-
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. - PubMed
-
- Barry SP, Townsend PA. What causes a broken heart—molecular insights into heart failure. Int Rev Cell Mol Biol. 2010;284:113–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

