Thin-filament length correlates with fiber type in human skeletal muscle
- PMID: 22075691
- PMCID: PMC3287155
- DOI: 10.1152/ajpcell.00299.2011
Thin-filament length correlates with fiber type in human skeletal muscle
Abstract
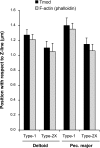
Force production in skeletal muscle is proportional to the amount of overlap between the thin and thick filaments, which, in turn, depends on their lengths. Both thin- and thick-filament lengths are precisely regulated and uniform within a myofibril. While thick-filament lengths are essentially constant across muscles and species (∼1.65 μm), thin-filament lengths are highly variable both across species and across muscles of a single species. Here, we used a high-resolution immunofluorescence and image analysis technique (distributed deconvolution) to directly test the hypothesis that thin-filament lengths vary across human muscles. Using deltoid and pectoralis major muscle biopsies, we identified thin-filament lengths that ranged from 1.19 ± 0.08 to 1.37 ± 0.04 μm, based on tropomodulin localization with respect to the Z-line. Tropomodulin localized from 0.28 to 0.47 μm further from the Z-line than the NH(2)-terminus of nebulin in the various biopsies, indicating that human thin filaments have nebulin-free, pointed-end extensions that comprise up to 34% of total thin-filament length. Furthermore, thin-filament length was negatively correlated with the percentage of type 2X myosin heavy chain within the biopsy and shorter in type 2X myosin heavy chain-positive fibers, establishing the existence of a relationship between thin-filament lengths and fiber types in human muscle. Together, these data challenge the widely held assumption that human thin-filament lengths are constant. Our results also have broad relevance to musculoskeletal modeling, surgical reattachment of muscles, and orthopedic rehabilitation.
Figures


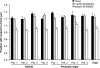

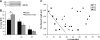

References
-
- Almenar-Queralt A, Lee A, Conley CA, Ribas de Pouplana L, Fowler VM. Identification of a novel tropomodulin isoform, skeletal tropomodulin, that caps actin filament pointed ends in fast skeletal muscle. J Biol Chem 274: 28466–28475, 1999 - PubMed
-
- Broschat KO. Tropomyosin prevents depolymerization of actin filaments from the pointed end. J Biol Chem 265: 21323–21329, 1990 - PubMed
-
- Broschat KO, Weber A, Burgess DR. Tropomyosin stabilizes the pointed end of actin filaments by slowing depolymerization. Biochemistry 28: 8501–8506, 1989 - PubMed
-
- Carlier MF, Valentin-Ranc C, Combeau C, Fievez S, Pantoloni D. Actin polymerization: regulation by divalent metal ion and nucleotide binding, ATP hydrolysis and binding of myosin. Adv Exp Med Biol 358: 71–81, 1994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

