Caspase-2: the orphan caspase
- PMID: 22075987
- PMCID: PMC3252831
- DOI: 10.1038/cdd.2011.157
Caspase-2: the orphan caspase
Abstract
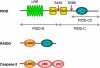
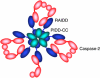
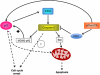
Despite an abundance of literature on the role of caspase-2 in apoptosis, there exists much controversy about this protease making it difficult to place caspase-2 correctly in the apoptotic cascade, and hence its role in apoptosis remains unclear. The identification of the PIDDosome as a signaling platform for caspase-2 activation prompted intense investigation into the true role of this orphan caspase. What has emerged is the idea that caspase-2 may not be mandatory for apoptosis and that activation of this caspase in response to some forms of stress has other effects on the cell such as regulation of cell cycle progression. This idea is particularly relevent to the discovery that caspase-2 may act as a tumor suppressor. Here, we discuss the proposed mechanisms through which caspase-2 signals, in particular those involving PIDD, and their impact on cellular fate.
Figures

References
-
- Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 2003;193:10–21. - PubMed
-
- Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 2002;9:358–361. - PubMed
-
- Vakifahmetoglu-Norberg H, Zhivotovsky B. The unpredictable caspase-2: what can it do. Trends Cell Biol. 2010;20:150–159. - PubMed
-
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

