Regulation of CD95/Fas signaling at the DISC
- PMID: 22075988
- PMCID: PMC3252827
- DOI: 10.1038/cdd.2011.155
Regulation of CD95/Fas signaling at the DISC
Abstract
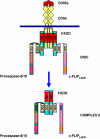
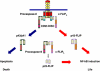
CD95 (APO-1/Fas) is a member of the death receptor (DR) family. Stimulation of CD95 leads to induction of apoptotic and non-apoptotic signaling pathways. The formation of the CD95 death-inducing signaling complex (DISC) is the initial step of CD95 signaling. Activation of procaspase-8 at the DISC leads to the induction of DR-mediated apoptosis. The activation of procaspase-8 is blocked by cellular FLICE-inhibitory proteins (c-FLIP). This review is focused on the role in the CD95-mediated signaling of the death effector domain-containing proteins procaspase-8 and c-FLIP. We discuss how dynamic cross-talk between procaspase-8 and c-FLIP at the DISC regulates life/death decisions at CD95.
Figures


References
-
- Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. - PubMed
-
- Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. - PubMed
-
- Weber CH, Vincenz C. The death domain superfamily: A tale of two interfaces. Trends Biochem Sci. 2001;26:475–481. - PubMed
-
- Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. - PubMed
-
- Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W, et al. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

