A supramolecular assembly formed by influenza A virus genomic RNA segments
- PMID: 22075989
- PMCID: PMC3300030
- DOI: 10.1093/nar/gkr985
A supramolecular assembly formed by influenza A virus genomic RNA segments
Abstract
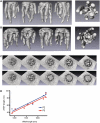
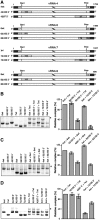
The influenza A virus genome consists of eight viral RNAs (vRNAs) that form viral ribonucleoproteins (vRNPs). Even though evidence supporting segment-specific packaging of vRNAs is accumulating, the mechanism ensuring selective packaging of one copy of each vRNA into the viral particles remains largely unknown. We used electron tomography to show that the eight vRNPs emerge from a common 'transition zone' located underneath the matrix layer at the budding tip of the virions, where they appear to be interconnected and often form a star-like structure. This zone appears as a platform in 3D surface rendering and is thick enough to contain all known packaging signals. In vitro, all vRNA segments are involved in a single network of intermolecular interactions. The regions involved in the strongest interactions were identified and correspond to known packaging signals. A limited set of nucleotides in the 5' region of vRNA 7 was shown to interact with vRNA 6 and to be crucial for packaging of the former vRNA. Collectively, our findings support a model in which the eight genomic RNA segments are selected and packaged as an organized supramolecular complex held together by direct base pairing of the packaging signals.
Figures






References
-
- Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 2005;3:591–600. - PubMed
-
- Palese P, Shaw M. Orthomyxoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott, Williams and Wilkins; 2006. pp. 1647–1689.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

