Apoptosis-inducing factor regulates skeletal muscle progenitor cell number and muscle phenotype
- PMID: 22076146
- PMCID: PMC3208607
- DOI: 10.1371/journal.pone.0027283
Apoptosis-inducing factor regulates skeletal muscle progenitor cell number and muscle phenotype
Abstract
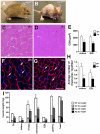
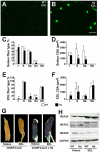
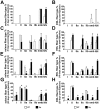
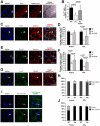
Apoptosis Inducing Factor (AIF) is a highly conserved, ubiquitous flavoprotein localized in the mitochondrial intermembrane space. In vivo, AIF provides protection against neuronal and cardiomyocyte apoptosis induced by oxidative stress. Conversely in vitro, AIF has been demonstrated to have a pro-apoptotic role upon induction of the mitochondrial death pathway, once AIF translocates to the nucleus where it facilitates chromatin condensation and large scale DNA fragmentation. Given that the aif hypomorphic harlequin (Hq) mutant mouse model displays severe sarcopenia, we examined skeletal muscle from the aif hypomorphic mice in more detail. Adult AIF-deficient skeletal myofibers display oxidative stress and a severe form of atrophy, associated with a loss of myonuclei and a fast to slow fiber type switch, both in "slow" muscles such as soleus, as well as in "fast" muscles such as extensor digitorum longus, most likely resulting from an increase of MEF2 activity. This fiber type switch was conserved in regenerated soleus and EDL muscles of Hq mice subjected to cardiotoxin injection. In addition, muscle regeneration in soleus and EDL muscles of Hq mice was severely delayed. Freshly cultured myofibers, soleus and EDL muscle sections from Hq mice displayed a decreased satellite cell pool, which could be rescued by pretreating aif hypomorphic mice with the manganese-salen free radical scavenger EUK-8. Satellite cell activation seems to be abnormally long in Hq primary culture compared to controls. However, AIF deficiency did not affect myoblast cell proliferation and differentiation. Thus, AIF protects skeletal muscles against oxidative stress-induced damage probably by protecting satellite cells against oxidative stress and maintaining skeletal muscle stem cell number and activation.
Conflict of interest statement
Figures


Similar articles
-
EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant.J Am Coll Cardiol. 2006 Aug 15;48(4):824-32. doi: 10.1016/j.jacc.2006.02.075. Epub 2006 Jul 25. J Am Coll Cardiol. 2006. PMID: 16904556
-
Downregulation of apoptosis-inducing factor in harlequin mutant mice sensitizes the myocardium to oxidative stress-related cell death and pressure overload-induced decompensation.Circ Res. 2005 Jun 24;96(12):e92-e101. doi: 10.1161/01.RES.0000172081.30327.28. Epub 2005 Jun 2. Circ Res. 2005. PMID: 15933268
-
Apoptosis-inducing factor regulates death in peripheral T cells.J Immunol. 2007 Jul 15;179(2):797-803. doi: 10.4049/jimmunol.179.2.797. J Immunol. 2007. PMID: 17617569
-
AIF, reactive oxygen species, and neurodegeneration: a "complex" problem.Neurochem Int. 2013 Apr;62(5):695-702. doi: 10.1016/j.neuint.2012.12.002. Epub 2012 Dec 12. Neurochem Int. 2013. PMID: 23246553 Free PMC article. Review.
-
Apoptosis-inducing factor: a matter of neuron life and death.Prog Neurobiol. 2007 Feb;81(3):179-96. doi: 10.1016/j.pneurobio.2006.12.002. Epub 2007 Jan 5. Prog Neurobiol. 2007. PMID: 17267093 Review.
Cited by
-
Physical Exercise and Mitochondrial Disease: Insights From a Mouse Model.Front Neurol. 2019 Jul 25;10:790. doi: 10.3389/fneur.2019.00790. eCollection 2019. Front Neurol. 2019. PMID: 31402893 Free PMC article.
-
Low-intensity microcurrent therapy promotes regeneration of atrophied calf muscles in immobilized rabbits.J Biomed Res. 2018 Nov 12;33(1):30-7. doi: 10.7555/JBR.32.20180056. Online ahead of print. J Biomed Res. 2018. PMID: 30418167 Free PMC article.
-
Genomic instability in the naturally and prematurely aged myocardium.Proc Natl Acad Sci U S A. 2021 Sep 7;118(36):e2022974118. doi: 10.1073/pnas.2022974118. Proc Natl Acad Sci U S A. 2021. PMID: 34465617 Free PMC article.
-
Magnetic resonance imaging at 7T reveals common events in age-related sarcopenia and in the homeostatic response to muscle sterile injury.PLoS One. 2013;8(3):e59308. doi: 10.1371/journal.pone.0059308. Epub 2013 Mar 12. PLoS One. 2013. PMID: 23555016 Free PMC article.
-
Apoptosis-inducing factor (AIF) at the crossroad of cell survival and cell death: implications for cancer and mitochondrial diseases.Cell Commun Signal. 2025 Jun 4;23(1):264. doi: 10.1186/s12964-025-02272-2. Cell Commun Signal. 2025. PMID: 40468311 Free PMC article. Review.
References
-
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. - PubMed
-
- Finkel T. Opinion: Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol. 2005;6:971–976. - PubMed
-
- Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–436. - PubMed
-
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. - PubMed
-
- Pak JW, Herbst A, Bua E, Gokey N, McKenzie D, et al. Mitochondrial DNA mutations as a fundamental mechanism in physiological declines associated with aging. Aging Cell. 2003;2:1–7. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

