Fascin is a key regulator of breast cancer invasion that acts via the modification of metastasis-associated molecules
- PMID: 22076152
- PMCID: PMC3208623
- DOI: 10.1371/journal.pone.0027339
Fascin is a key regulator of breast cancer invasion that acts via the modification of metastasis-associated molecules
Abstract
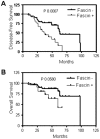
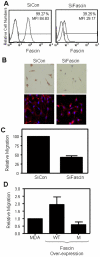
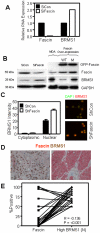
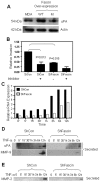
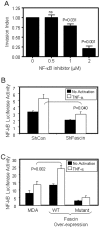
The actin-bundling protein, fascin, is a member of the cytoskeletal protein family that has restricted expression in specialized normal cells. However, many studies have reported the induction of this protein in various transformed cells including breast cancer cells. While the role of fascin in the regulation of breast cancer cell migration has been previously shown, the underlying molecular mechanism remained poorly defined. We have used variety of immunological and functional assays to study whether fascin regulates breast cancer metastasis-associated molecules. In this report we found a direct relationship between fascin expression in breast cancer patients and; metastasis and shorter disease-free survival. Most importantly, in vitro interference with fascin expression by loss or gain of function demonstrates a central role for this protein in regulating the cell morphology, migration and invasion potential. Our results show that fascin regulation of invasion is mediated via modulating several metastasis-associated genes. We show for the first time that fascin down-regulates the expression and nuclear translocation of a key metastasis suppressor protein known as breast cancer metastasis suppressor-1 (BRMS1). In addition, fascin up-regulates NF-kappa B activity, which is essential for metastasis. Importantly, fascin up-regulates other proteins that are known to be critical for the execution of metastasis such as urokinase-type plasminogen activator (uPA) and the matrix metalloproteases (MMP)-2 and MMP-9. This study demonstrates that fascin expression in breast cancer cells establishes a gene expression profile consistent with metastatic tumors and offers a potential therapeutic intervention in metastatic breast cancer treatment through fascin targeting.
Conflict of interest statement
Figures

References
-
- Bashyam MD. Understanding cancer metastasis: an urgent need for using differential gene expression analysis. Cancer. 2002;94:1821–1829. - PubMed
-
- Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243–255, vii. - PubMed
-
- Fidler IJ. Seed and soil revisited: contribution of the organ microenvironment to cancer metastasis. Surg Oncol Clin N Am. 2001;10:257–269. vii-viiii. - PubMed
-
- Nicolson GL. Organ specificity of tumor metastasis: role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev. 1988;7:143–188. - PubMed
-
- Saaristo A, Karpanen T, Alitalo K. Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis. Oncogene. 2000;19:6122–6129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

