Measurement and correction of in vivo sample aberrations employing a nonlinear guide-star in two-photon excited fluorescence microscopy
- PMID: 22076274
- PMCID: PMC3207382
- DOI: 10.1364/BOE.2.003135
Measurement and correction of in vivo sample aberrations employing a nonlinear guide-star in two-photon excited fluorescence microscopy
Abstract
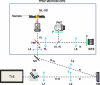
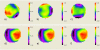
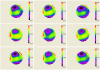
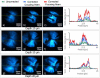
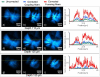
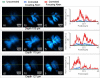
We demonstrate that sample induced aberrations can be measured in a nonlinear microscope. This uses the fact that two-photon excited fluorescence naturally produces a localized point source inside the sample: the nonlinear guide-star (NL-GS). The wavefront emitted from the NL-GS can then be recorded using a Shack-Hartmann sensor. Compensation of the recorded sample aberrations is performed by the deformable mirror in a single-step. This technique is applied to fixed and in vivo biological samples, showing, in some cases, more than one order of magnitude improvement in the total collected signal intensity.
Keywords: (170.3880) Medical and biological imaging; (180.4315) Nonlinear microscopy; (190.4180) Multiphoton processes; (220.1000) Aberration compensation; (220.1080) Active or adaptive optics.
Figures

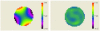

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
